
Introduction
Transient scaffolding using bioresorbable drug-eluting polymeric platforms aims to improve late clinical outcomes through restoration of vasomotion, adaptive shear stress, late plaque regression and expansive remodelling. However, long-term data are scarce. Several studies have reported the midterm comparison of the Absorb™ bioresorbable scaffold (Abbott Vascular, Santa Clara, CA, USA) with its metallic stent counterpart, XIENCE® (Abbott Vascular)1,2. Preclinical evaluation has shown that scaffold degradation is complete by 36 months3. The aim of this report is to present the first four-year comparative evaluation after the expected full resorption of the scaffold device.
Methods
The trial design and methods, as well as the study population, were described in previous reports4,5. An amendment was appended to the initial trial design to extend the clinical follow-up beyond the three-year primary endpoint timeline up to five years; twenty patients refused to re-consent to this extended follow-up (nine in the Absorb arm and 11 in the XIENCE arm). A single four-year follow-up visit was performed in 86% (Absorb) and 84% (XIENCE) of the initial cohorts. Reasons for missing evaluation are described in the flow chart (Figure 1). All clinical events were adjudicated by an independent clinical events committee. Fisher’s exact test was used to compare binary variables of clinical outcome. For time-to-event variables, survival curves were constructed using Kaplan-Meier estimates and the log-rank test was used.
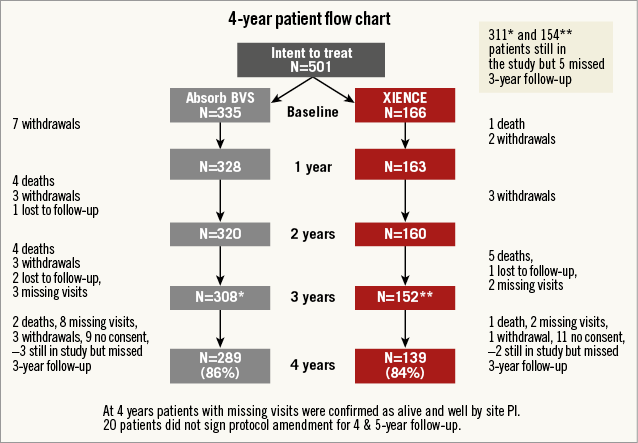
Figure 1. Study flow chart. *Absorb BVS group; **XIENCE group; PI: principal investigator
Results
The endpoints of the three-year angiographic follow-up were reported in a previously published 3-year clinical comparative study1. Between three and four years, target lesion failure (or the device-oriented composite endpoint [DOCE]) increased from 10.5% to 11.5% in the Absorb arm and from 5.0% to 6.0% in the XIENCE arm, with 1% and 0.7% absolute difference, respectively (p=0.3). No statistically significant difference could be observed (p=0.063) (Figure 2). Two patients in the Absorb arm and one in the XIENCE arm died between three and four years: causes of death were one sudden death in each arm and one brain tumour in the Absorb group. The patient-oriented composite endpoint (POCE) was observed in 23.6% in the Absorb arm and 26.7% in the XIENCE arm (p=0.47). The individual components of the composite endpoints and non-hierarchical analyses of the clinical events are presented in Table 1. No case of additional very late scaffold/stent thrombosis was noted in either arm between three and four years, with a four-year rate of 3.0% versus 0.0% (p=0.035) (Figure 3). DAPT prescription slightly decreased from 29.8% to 25.9% in the Absorb arm and from 27.7% to 21.1% in the XIENCE arm, with no significant difference between the two arms.
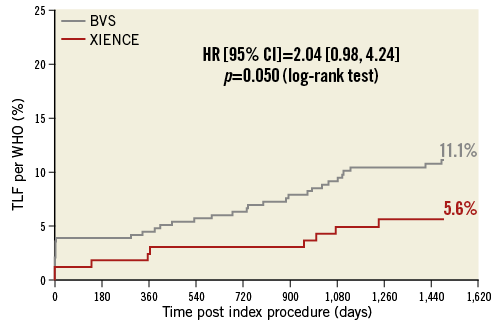
Figure 2. Target lesion failure up to four years.
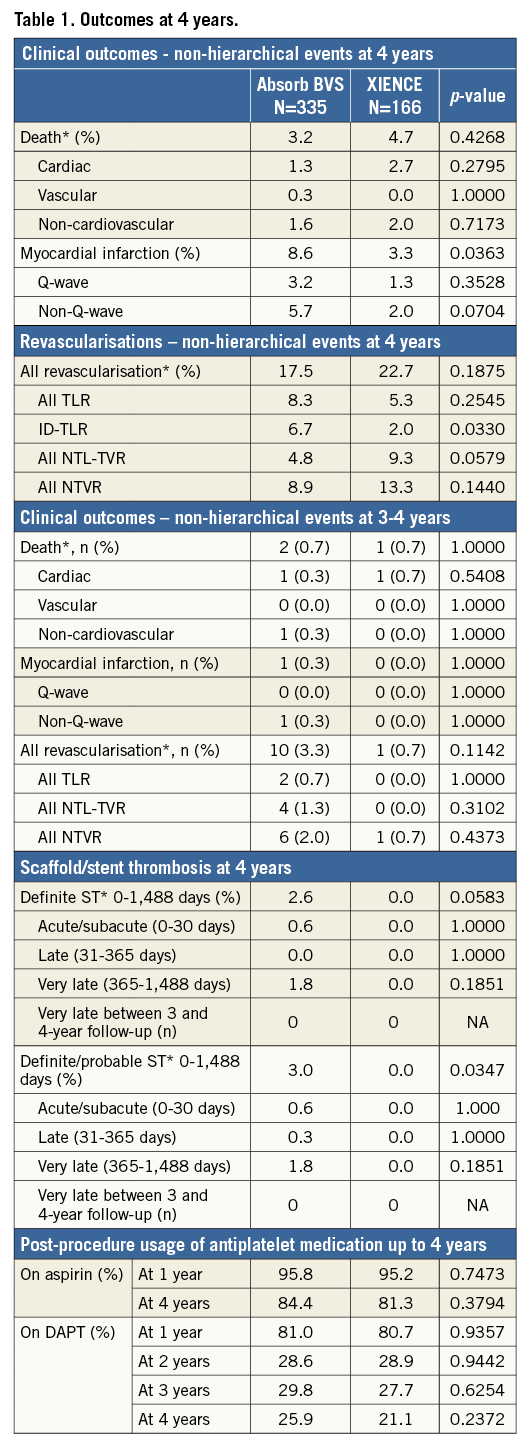

Figure 3. Stent thrombosis up to four years. ARCST DPr: Stent thrombosis adjudicated as definite/probable according to the Academic Research Consortium definitions
Discussion
This first description of the four-year follow-up of a randomised comparison between Absorb and XIENCE showed a similarly low rate of additional target lesion failure (TLF) events in both groups after three years without additional instances of scaffold thrombosis despite the absence of DAPT in three quarters of the cohort. Very late scaffold thrombosis during the third year was the main contributor to the significant difference in TLF rates at three years. A downturn of events has now been observed between three and four years, impacting positively on the TLF difference at four years. Intraluminal dismantling or late discontinuities of the scaffold have been proposed as possible mechanisms of this peak of thrombosis between 32 and 36 months after implantation as described in case reports6 and in the ABSORB Japan trial7. A similar downturn was observed in terms of the POCE after a peak of events at 36 months which is likely to be related to systematic angiographic follow-up inducing non-TLR revascularisations. Scaffold sizing and implantation technique have a key impact on three-year clinical results, as shown in a recent meta-analysis and a report by Serruys et al8,9. The impact of these factors, as well as DAPT prolongation, on longer-term outcomes is still unknown and needs to be demonstrated.
Limitations
Further long-term follow-up data from larger studies are necessary to overcome the two main limitations of the ABSORB II study: 1) the trial was not powered to evaluate clinical endpoints; 2) the implantation technique reflects the state of the art at the time of enrolment in 2011-2012 and did not take into account the more recent findings on bioresorbable scaffold usage in terms of size selection, lesion preparation and deployment technique.
Conclusion
A downturn of events was observed beyond the expected resorption time. Further long-term evaluation in larger randomised studies is necessary to confirm this observation.
| Impact on daily practice Previous midterm follow-up reports after Absorb implantation have shown an increase of scaffold thrombosis leading to an excess of target lesion failure. The four-year follow-up of the ABSORB II Trial is the first observation of clinical outcome after the expected resorption time in the setting of a randomised clinical trial. No additional thrombosis was observed between three and four years. The implications for long-term routine follow-up and dual antiplatelet therapy duration may need additional information from larger trials and meta-analysis of ABSORB studies. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat, Paris, and University Paris VII, Paris, France.
Funding
The ABSORB II Trial (NCT01425281) was funded by Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
P. Serruys is a member of the Advisory Board of Abbott Vascular. B. Chevalier was a consultant for Abbott Vascular. Y. Onuma is a member of the Advisory Board for Abbott Vascular and has received speaker honoraria from Terumo. M. Sabate is a consultant for Abbott Vascular. D. Dudek is a member of the Advisory Board and receives research and education support from Abbott. The other authors have no conflicts of interest to declare. The Guest Editor declares receiving consultancy fees from Edwards Lifesciences.




