Introduction
Over 43 million people in the United States (US) have contracted COVID-19, resulting in ~698,000 deaths [1]. The surges in cases have brought with them over 3,000,000 hospitalizations that threaten to overwhelm the strained healthcare system [1]. One approach to reducing the impact of COVID-19 on the healthcare system is through early treatment of patients in the outpatient setting.
The monoclonal antibody products bamlanivimab [2] and casirivimab/imdevimab [3] recently received emergency use authorization from the Food and Drug Administration (FDA) for the treatment of COVID-19 patients at high risk for progressing to hospitalization. While these early treatment trials were positive, bamlanivimab was found inefficacious for the treatment of hospitalized COVID-19 patients [4], supporting the hypothesis that antiviral therapy is most effective early in the course of disease [5].
The results of hydroxychloroquine randomized controlled trials (RCTs) and cohort studies for the treatment of hospitalized patients have been lackluster [6, 7] and its emergency use authorization was revoked by the FDA [8]. However, at the November 2020 American Medical Association meeting, delegates tried to get the organization to revoke its statement discouraging the use of hydroxychloroquine for COVID-19, especially for the early outpatient treatment of the disease [9]. That same month, several panelists at a US Senate hearing touted hydroxychloroquine’s outpatient use in COVID-19, and asked for its emergency use authorization to be reinstated [10]. If hydroxychloroquine is effective and safe in the early treatment of COVID-19, it would be markedly less expensive than monoclonal antibody therapy and much more readily available to roll out to the general public. However, the use of hydroxychloroquine may have adverse events and shunting utilization to COVID-19 patients could cause shortages for those patients who need hydroxychloroquine for autoimmune diseases and malaria [11].
In this systematic review with meta-analyses, we assessed the efficacy and safety of hydroxychloroquine in early onset treatment of COVID-19 from all the available randomized controlled trials.
Material and methods
Data sources and searches
Three investigators (C.M.W., V.P., and A.V.H.) developed the search strategy, which was revised and approved by the other investigators. We searched the following databases from December 1, 2019 to September 14, 2021: PubMed-MEDLINE, EMBASE-OVID, Scopus, Web of Science, the Cochrane Library, bioRxiv (www.biorxiv.org), Preprints (www.preprints.org), Clinical Trials.gov, the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/), and the Chinese Clinical Trials Registry (www.chictr.org.cn). The PubMed search strategy is shown in the Supplementary file.
Study selection
We included randomized controlled studies (RCTs) in any language reporting benefit or harm outcomes from use of hydroxychloroquine as early treatment (i.e. a few days from symptom onset to enrolment) in outpatients with mild to moderate reverse transcription-polymerase chain reaction (RT-PCR)-confirmed COVID-19. We excluded studies in hospitalized COVID-19 patients, even though patients had mild to moderate disease and/or early disease, studies of prophylaxis with hydroxychloroquine (i.e. in those without COVID-19), and cohort studies evaluating hydroxychloroquine as early treatment of COVID-19. Three investigators (A.V.H., V.P., Y.M.R.) independently screened each record title and abstract for potential inclusion. Three investigators (V.P., J.J.B., Y.M.R.) then read the full text of the records whose abstracts had been selected by at least one investigator. Discrepancies were resolved through discussion or by a fourth investigator (A.V.H.).
Outcomes
Primary outcomes were hospitalization and all-cause mortality. Secondary outcomes were intensive care unit (ICU) admission, need of mechanical ventilation, COVID-19 symptom resolution, viral clearance in nasopharyngeal swabs, adverse events, and specific adverse events (e.g. diarrhea, headache, QTc prolongation).
Data extraction
Two investigators (A.P., J.J.B.) independently extracted the following variables from studies: study setting, country, mean age, proportion of males, time from symptom onset in days, proportion of chronic coexisting diseases, hydroxychloroquine dose and duration, type of control and description, additional drug interventions, primary and secondary outcomes, and time of follow-up. Discrepancies were resolved through discussion or by a third investigator (A.V.H.).
Risk of bias assessment
Two investigators (A.P., J.J.B.) independently assessed risk of bias (RoB) of randomized controlled trials with the Cochrane Risk of Bias 2.0 tool for RCTs [12, 13]; disagreements were resolved by discussion with a third investigator (A.V.H.). RoB 2 assesses five domains: bias due to the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. RoB of each domain and each RCT was described as low, some concerns or high.
Statistical analysis
We reported our systematic review according to 2009 PRISMA guidelines [14]. Inverse variance random effect meta-analyses were performed to evaluate effect of hydroxychloroquine vs. control on outcomes when outcome data were available for at least two RCTs or cohorts judged to have homogeneous study characteristics. Effects of meta-analyses were reported as relative risks (RR) for dichotomous outcomes and as mean differences (MD) for continuous outcomes, along with their 95% confidence intervals (CIs). CIs of effects were adjusted with the Hartung-Knapp method [15], and the between study variance τ2 was calculated with the Paule-Mandel method. Heterogeneity of effects among studies was quantified with the I2 statistic (I2 > 60% means high heterogeneity). The meta package of R 3.5.1 (www.r-project.org) was used for meta-analyses. The quality of evidence (QoE) was evaluated using the GRADE methodology, which covers five aspects: risk of bias, inconsistency, indirectness, imprecision, and publication bias [16]. Quality of evidence was evaluated per outcome and described in summary of findings (SoF) tables; GRADEpro GDT was used to create SoF tables [17].
Results
Selection of studies
Our comprehensive search yielded 9551 citations with an additional 927 citations identified through other sources, including backwards citation tracking. After removing duplicates and applying our inclusion and exclusion criteria (Supplementary Figure S1), we identified five RCTs [18–22] (n = 1848) which were all homogeneous enough to warrant meta-analyses.
Characteristics of included studies
The general characteristics of the included RCTs are included in Table I. Placebo was the comparator in four RCTs [19–22] while usual care was the comparator in the open label one [18]. The five RCTs used hydroxychloroquine total doses between 1,600 and 4,400 mg and had follow-up times between 14 and 90 days. Mean or median ages ranged between 37 and 53 years, males between 31% and 55%, median time of COVID-19 symptom onset between 3 and 7 days, with most individuals having symptom onset within 9 days, and the proportion of individuals without coexisting disease between 36% and 68%.
Table I
Baseline characteristics of randomized controlled studies included in the systematic review
| Author, year [ref.]/type of study/registration | Objective | Sample, country | Population | Overall key patient characteristics | Intervention | Comparison | Outcomes | Follow-up time |
|---|---|---|---|---|---|---|---|---|
| Mitjà et al., 2020 [18], Parallel RCT, NCT04304053 | To determine whether early treatment with HCQ would be more efficacious than no treatment for outpatients with mild COVID-19 | 293 (I: 136, C: 157), Spain | Adult (≥ 18 years) patients who had mild symptoms of COVID-19 (i.e., fever, acute cough, shortness of breath, sudden olfactory or gustatory loss, or influenza-like-illness) for < 5 days before enrollment, were non-hospitalized, and had a positive PCR test for SARS-CoV-2 in the baseline NP swab | Mean (SD) age: 41.6 (12.6) years Male: 31% Median (IQR) time from symptom onset to enrolment: 3 (2–4) days No coexisting disease: 47% | HCQ 800 mg on day 1, followed by 400 mg once daily for 6 days (3,200 mg total dose). Initially, the protocol used HCQ plus cobicistat-boosted darunavir (DRVc) combined treatment, but it was adapted to HCQ alone | Usual care (no details provided) | Primary: Viral RNA load in NP swabs at days 3, and 7 after treatment start Secondary: Clinical progression with simplified version of WHO progression scale of 4 points, time from randomization to complete resolution of symptoms within 28 days, resolution of symptoms, severe adverse events | 28 days |
| Skipper et al. 2020 [19], Parallel RCT, NCT0408668 | To investigate whether HCQ could reduce COVID-19 severity in adult outpatients | 491 (I: 212, C: 211), USA and Canada | Non-hospitalized adults (≥ 18 years) with ≤ 4 days of symptoms and either PCR-confirmed SARS-CoV-2 infection or symptoms after a high-risk exposure to a PCR-confirmed COVID-19 person within the past 14 days. HCW who had COVID-19 symptoms and high-risk exposure but whose contact had PCR results pending. Participants with a high-risk exposure and asymptomatic at the time of consent of accompanying prophylaxis RCT; these became symptomatic before day 1 | Median (IQR) age: 40 (32–50) years Male: 44% Proportion with duration of symptoms onset to enrolment ≤ 2 days: 74% No coexisting disease: 68% COVID-19 PCR positive: 34% HCW: 57% | HCQ 800 mg (4 tablets) once, then 600 mg 6 to 8 h later, then 600 mg once daily for 4 more days (5 days in total) (3,800 mg total dose) | Placebo tablets of folic acid (400 μg) prescribed as an identical regimen | Original primary: Ordinal outcome by day 14 of not hospitalized, hospitalized, or ICU stay or death. Modified primary: Change in overall symptom severity over 14 days measured on a 10-point VAS Secondary: Symptom severity at day 5 and day 14 by 10-point VAS, incidence of all hospitalizations and deaths, and incidence of study medicine withdrawal | 14 days |
| Johnston et al. 2021 [20], cluster RCT, NCT04354428 | To evaluate the efficacy of HCQ and HCQ+AZ to prevent progression of COVID-19 among high- and low-risk outpatients with COVID-19 | 231 (HCQ:71, HCQ + AZ: 77, P: 83), USA | Age between 18 and 80 years, lab-confirmed SARS-CoV-2 infection within the prior 72 h. High-risk group: established risk factors for severe COVID-19 (age ≥ 60, pulmonary disease, DM, HTN, BMI ≥ 30). Low risk group: did not meet any high-risk criteria | Median (min.–max.) age: 37 (18-78) years Male: 43% Median (IQR) time from symptom onset to enrolment: 5.9 (4–8.2) days No coexisting disease (low risk group): 44% | HCQ 400 mg day 1, then 200 mg twice daily for 9 days (4,000 mg total dose) HCQ 400 mg + AZ 500 mg day 1, then HCQ 200 mg twice daily for 9 days + AZ 250 mg once daily for 4 days (HCQ 4,000 mg total dose + AZ 1,500 total dose) | Placebo-equivalent (ascorbic acid (500 mg day 1, 250 mg twice daily for 9 days) + folic acid (800 μg day 1, 400 μg twice daily for 4 days)) | Primary: Composite of 14-day development of LRTI (SpO2 < 93% on two readings with symptoms), 28-day COVID-19 related hospitalization or death; 14-day time to viral clearance Secondary: Time to symptom resolution by day 14 among those who had COVID-19 symptoms at baseline; adverse events | 28 days |
| Reis et al. 2021 [21], parallel RCT, NCT04403100 | To determine whether HCQ or L/R reduces hospitalization among high-risk patients with early symptomatic COVID-19 in an outpatient setting | 685 (HCQ: 214, L/R: 244, P: 227), Brazil | 18 years or older, reported < 8 days since onset of flulike symptoms or chest CT scan consistent with COVID-19, lab-confirmed SARS-CoV-2 infection, and at least one criterion for high risk: ≥ 50 years, pulmonary disease (moderate or severe asthma, COPD, pulmonary HTN, or emphysema), DM, HTN, known CVD, BMI ≥ 30, immunocompromised status, cancer | Median (IQR) age: 53 (18–94) years Male: 45% Proportion with time since onset of symptoms ≤ 5 days: 16% Only one risk factor for high-risk disease: 43% | HCQ 800 mg day 1, then 400 mg for 9 days (4,400 mg total dose) L/R 800/200 mg first two intakes, then 400/100 mg twice a day for 9 days (5,200/1,300 mg total dose) | Placebo (inert material – talc); bottles were identical to HCQ or L/R | Primary: COVID-associated hospitalization; death. Both measured at 90 days Secondary: Hospital admission for any cause; proportion of persons with clearance of SARS-CoV-2 at days 3, 7, and 14; time to symptom resolution; treatment emergent adverse events | 90 days |
| Schwartz et al. 2021 [22], parallel RCT, NCT04329611 | To determine whether HCQ treatment for outpatients with SARS-CoV-2 infection could prevent hospitalization, mechanical ventilation or death | 148 (HCQ: 111, P: 37), Canada | Adults with SARS-CoV-2 infection confirmed by RT-PCR from a NP or pharyngeal swab within the previous 4 days, with symptom onset within the previous 12 days, and with ≥ 1 risk factor for severe disease (receiving immunosuppressants or biologic therapies, age ≥ 40, BMI > 40, chronic lung disease, HTN, DM, CVD, CKD, cancer, transplant recipient, severe immune suppression, smoking) | Mean (SD) age: 46.8 (11.3) years Male: 55% Median (IQR) time from symptom onset to randomization: 7 (5–9) days Low risk (age 40–64 y with no other risk factors): 36% | HCQ 800 mg day 1, then 200 mg twice daily for 4 days (1,600 mg total dose) | Matching placebo (12 tablets over 5 days) | Primary: Severe disease (composite of hospitalization, invasive mechanical ventilation or death within 30 days) Secondary: Days to symptom resolution; disposition at 30 days (recovered, symptomatic non-hospitalized, hospitalized, admitted to ICU, dead); serious adverse events | 30 days |
[i] COVID-19 – coronavirus disease 2019, I – intervention, C – comparator, P – placebo, HCQ – hydroxychloroquine, AZ – azithromycin, L/R – lopinavir/ritonavir, NP – nasopharyngeal, CT – computed tomography, HCW – health care worker, RT-PCR – reverse transcriptase polymerase chain reaction, ICU – intensive care unit, VAS – visual analogue scale, SC – standard of care, NA – not available; min. – minimum, max. – maximum, DM – diabetes mellitus, HTN – hypertension, COPD – chronic obstructive pulmonary diseases, CVD – cardiovascular diseases, CKD – chronic kidney disease, BMI – body mass index, LRTI – lower respiratory tract infection.
Risk of bias of included studies
One RCT had high risk of bias due to missing outcome data [19], two RCTs had some concerns of bias due to deviations from intended interventions and selection in the reported result [18] and due to bias in the randomization process [21], and two RCTs had low risk of bias [20, 22] (Supplementary Figure S2).
Effects of early treatment with hydroxichloroquine on outcomes
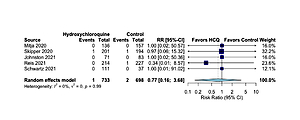
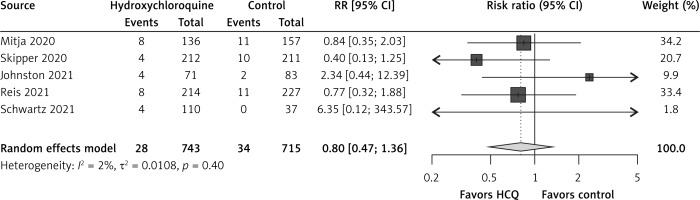
In comparison to the control group, hydroxychloroquine non-significantly reduced hospitalizations by 20% (RR = 0.80, 95% CI: 0.47–1.36, I2 = 2%, 5 RCTs, low QoE, Figure 1) and all-cause mortality by 23% (RR = 0.77, 95% CI: 0.16–3.68, I2 = 0%, 5 RCTs, very low QoE, Figure 2). Also, hydroxychloroquine had no effect on COVID-19 symptom resolution (RR = 0.94, 95% CI: 0.77–1.16, I2 = 71%, 3 RCTs, low QoE, Supplementary Figure S3), time to COVID-19 symptom resolution (MD = –0.16 days, 95% CI: –4.56 to 4.25 days, I2 = 80%, 2 RCTs, very low QoE, Supplementary Figure S4) or on viral clearance at 14 days (RR = 1.02, 95% CI: 0.82–1.27, I2 = 65%, 2 RCTs, low QoE, Supplementary Figure S5) in comparison to the control group.
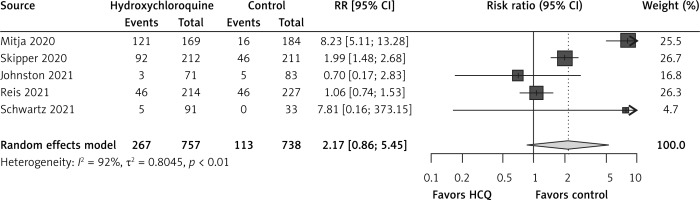
There were no data about ICU admissions or need of mechanical ventilation in RCTs. Hydroxychloroquine non-significantly increased risks of adverse events in comparison to the control group (RR = 2.17, 95% CI: 0.86-5.45, I2 = 92%, 5 RCTs, very low QoE, Figure 3). Reports of specific adverse events in the RCTs were very scarce.
Quality of evidence of effects
The quality of evidence was low to very low for all outcomes (Table II). The main drivers of poor quality of evidence in RCTs were high risk of bias, imprecision of effects and inconsistency.
Table II
Summary of findings table for the effects of early treatment with hydroxychloroquine vs. control in COVID-19 patients
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with control | Risk with hydroxychloroquine | ||||
| Hospitalization follow-up: range 14 days to 90 days | 5 per 100 | 4 per 100 (2 to 6) | RR = 0.80 (0.47 to 1.36) | 1458 (5 RCTs) | ⊕⊕○○ Lowa |
| All-cause mortality follow-up: range 14 days to 90 days | 0 per 100 | 0 per 100 (0 to 1) | RR = 0.77 (0.16 to 3.68) | 1431 (5 RCTs) | ⊕○○○ Very lowa,b |
| COVID-19 symptom resolution follow-up: range 14 days to 30 days | 67 per 100 | 63 per 100 (51 to 77) | RR = 0.94 (0.77 to 1.16) | 675 (3 RCTs) | ⊕⊕○○ Lowc,d |
| Time to COVID-19 symptom resolution assessed with: days follow-up: range 28 days to 30 days | The mean time to COVID-19 symptom resolution was 12.7 days | MD 0.16 days lower (4.56 lower to 4.25 higher) | – | 417 (2 RCTs) | ⊕○○○ Very lowe,f,g |
| Viral clearance assessed with: RT-PCR from nasopharyngeal swab follow-up: mean 14 days | 62 per 100 | 63 per 100 (50 to 78) | RR = 1.02 (0.82 to 1.27) | 481 (2 RCTs) | ⊕⊕○○ Lowh,i |
| Adverse events follow-up: range 14 days to 28 days | 15 per 100 | 33 per 100 (13 to 83) | RR = 2.17 (0.86 to 5.45) | 1495 (5 RCTs) | ⊕○○○ Very lowa,j,k |
a Very serious risk of bias due to high risk of bias in Skipper 2020 due to missing outcome data, and some concerns of bias in Mitja 2020 due to deviations from intended interventions and selection of the reported results and in Reis 2021 due to bias in the randomization process.
Discussion
In our systematic review we found that hydroxychloroquine as early treatment for COVID-19 was not associated with lower hospitalization, all-cause mortality, or overall adverse events risks vs. controls (usual care or placebo) in five RCTs. There was no effect of hydroxychloroquine on COVID-19 symptom resolution or viral clearance at 14 days. No data on other outcomes such as ICU admissions, need for mechanical ventilation, or specific adverse events were reported. The quality of evidence was low to very low in all outcomes.
The 20% relative reductions in hospitalizations in RCTs is encouraging, especially since three of the biggest RCTs had the same direction of effect in favor of hydroxychloroquine, but none of the individual risks was significant across RCTs. If this were a real benefit of therapy, reducing relative risks of hospitalizations in those recently contracting COVID-19 by one fifth would make a difference in the overstressed healthcare system. However, more RCTs of higher methodologic quality are needed for us to adequately assess this outcome with the use of early treatment with hydroxychloroquine in the future.
The RCTs found no impact on all-cause mortality; probable reasons included scarcity of events (i.e. one event per arm in Skipper et al. [19] and one event in the control arm in Reis et al. [21]), and also short time of follow-up as Skipper et al. [19] only had 14 days, Mitjà et al. [18] and Johnston et al. [20] only had 28 days, and Schwartz et al. [22] only had 30 days, so it could have been too soon to see mortality reductions. The dose of hydroxychloroquine therapy was not a viable explanation for the lack of effect on all-cause mortality across RCTs as the total doses in the RCTs ranged from 3,200 mg to 4,400 mg, with the exception of the small RCT by Schwartz et al. [22] with a total dose of 1,600 mg. The duration of hydroxychloroquine was not a viable explanation either, with RCTs providing hydroxychloroquine therapy for 5 to 9 days. Three recent systematic reviews and meta-analyses including RCTs until October 16, 2020 did not evaluate mortality effects of hydroxychloroquine in outpatients [23–25]; two other recent systematic reviews and meta-analyses including RCTs until October 15, 2020 [26, 27] only assessed mortality effects in COVID-19 outpatients using the Mitja et al. [18] and Skipper et al. [19] RCTs.
Hydroxychloroquine for the early treatment of COVID-19 would compete against the monoclonal antibody products bamlanivimab [2] and casirivimab/imdevimab [3] that recently received emergency use authorization from the FDA for the treatment of COVID-19 patients at high risk for progressing to hospitalization. These drugs will cost between $1,250 and $1,500 per dose according to governmental contracts. Bamlanivimab was authorized based on the results from the ‘Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies’ (BLAZE-1) RCT [28]. In the combined bamlanivimab dosing group, the incidence of hospitalizations or emergency department visits was non-significantly lower (3 of 309 (1.6%) vs. 9 of 143 (6.3%)) by day 29. In a 799-patient trial that is still unpublished [29], treatment with casirivimab/imdevimab was associated with a 57% reduction in COVID-19-related medical visits until day 29, which was significantly greater than the placebo group (p = 0.024).
The National Institutes of Health COVID-19 Treatment Guideline Panel [2] has cautioned that there is insufficient evidence of bamlanivimab’s efficacy in early outpatient treatment and has not commented on the use of casirivimab/imdevimab because the data are unpublished. As such, we cannot determine whether any of these therapies is truly beneficial in the early treatment of COVID-19 or whether one option is superior to the others. If hydroxychloroquine is subsequently found to significantly reduce hospitalizations, it would offer several advantages over monoclonal antibody therapy including a better established safety profile, lower acquisition cost, more convenient dosing form, and more ample supply for COVID-19 patients.
However, if hydroxychloroquine is not truly efficacious or effective, the adverse events associated with therapy would not be balanced out with benefits, and shunting the drug supply away from patients with autoimmune diseases could negatively impact other patients in the healthcare system. In a study of 3,872 patients taking hydroxychloroquine or chloroquine for autoimmune diseases [11], 27%, 21%, 7%, and 2% of patients in Africa, South-East Asia, North and South America and Europe, respectively, reported running out of medication due to drug shortages in the COVID-19 era. These patients experienced poorer physical (5.6 < 6.4, t(254) = 5.97, p < 0.001) and mental (5.8 < 6.3, t(252) = 3.82, p < 0.001) health and higher levels of rheumatic disease activity (5.1 > 4.3, t(244) = 4.44, p < 0.001) as a result.
Our study has several limitations. First, all outcomes had low to very low quality of evidence mainly explained by heterogeneity of effects across trials, high risk of bias or some concerns of bias of three RCTs, and imprecision of effects. Second, there were very scarce all-mortality data across RCTs, and only two or three RCTs had information on symptom resolution or viral clearance. Third, there were no data on ICU admissions, need of mechanical ventilation, or specific adverse events. Fourth, four of the five RCTs [19–22] had fewer patients randomized than originally planned; this may have resulted in lack of power to detect effects of hydroxychloroquine on outcomes. Finally, we did not evaluate the effect of adding azithromycin to hydroxychloroquine in our study.
In conclusion, hydroxychloroquine as early treatment did not reduce hospitalizations, all-cause mortality, COVID-19 symptom resolution, or viral clearance in COVID-19 outpatients from five RCTs in comparison to placebo or usual care. There also was a non-significant increase in adverse events with hydroxychloroquine as early treatment. There were no data for outcomes such as ICU admission, need of mechanical ventilation or specific adverse events. The quality of evidence was low to very low for all outcomes.
Given its low acquisition cost, relative safety, convenient administration route, and available supply, hydroxychloroquine should continue to be investigated for outpatients who test positive for COVID-19. However, hydroxychloroquine should not be recommended for acute treatment at this time because the balance of benefits to harms cannot be determined given the current literature base.