Published online Aug 24, 2021. doi: 10.5306/wjco.v12.i8.581
Peer-review started: December 24, 2020
First decision: May 4, 2021
Revised: May 12, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 24, 2021
Malignant pleural mesothelioma (MPM) is a rare tumor with poor prognosis and rising incidence. Palliative care is common in MPM as radical treatment with curative intent is often not possible due to metastasis or extensive locoregional involvement. Numerous therapeutic advances have been made in recent years, including the use of less aggressive surgical techniques associated with lower morbidity and mortality (e.g., pleurectomy/decortication), technological advancements in the field of radiotherapy (intensity-modulated radiotherapy, image-guided radiotherapy, stereotactic body radiotherapy, proton therapy), and developments in systemic therapies (chemotherapy and immunotherapy). These improvements have had as yet only a modest effect on local control and survival. Advances in the management of MPM and standardization of care are hampered by the evidence to date, limited by high heterogeneity among studies and small sample sizes. In this clinical guideline prepared by the oncological group for the study of lung cancer of the Spanish Society of Radiation Oncology, we review clinical, histologic, and therapeutic aspects of MPM, with a particular focus on all aspects relating to radiotherapy, including the current evidence base, associations with chemotherapy and surgery, treatment volumes and planning, technological advances, and reradiation.
Core Tip: Malignant pleural mesothelioma is a rare tumor that is very challenging to treat. Technological advances in surgery and radiotherapy are largely responsible for the marginally improved outcomes observed in recent years. Heterogeneity among studies and a lack of phase III randomized controlled trials are some of the main barriers to achieving more effective, standardized treatments. In this review article, we provide an in-depth analysis of changes in the clinical, histologic, and therapeutic profile of malignant pleural mesothelioma in recent decades and highlight the main research areas in this field.
- Citation: Luna J, Bobo A, Cabrera-Rodriguez JJ, Pagola M, Martín-Martín M, Ruiz MÁG, Montijano M, Rodríguez A, Pelari-Mici L, Corbacho A, Moreno M, Couñago F. GOECP/SEOR clinical guidelines on radiotherapy for malignant pleural mesothelioma. World J Clin Oncol 2021; 12(8): 581-608
- URL: https://www.wjgnet.com/2218-4333/full/v12/i8/581.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i8.581
Malignant pleural mesothelioma (MPM) is a rare tumor, but it is a global health concern because of its poor prognosis and rising incidence.
Improvements in our understanding of MPM pathogenesis[1,2], together with encouraging results from recent studies of multimodality therapies, targeted therapies, and immunotherapies have brought new hope for the management of this disease[3].
Optimal treatment strategies have not been defined for MPM. In these guidelines, drawn up by the Oncological Group for the Study of Lung Cancer of the Spanish Society of Radiation Oncology, we review the current status and prospects of MPM management with a focus on all aspects relating to radiotherapy.
Incidence: MPM incidence is strongly associated with asbestos exposure[4,5], but there is a long latency period, meaning that in some cases, the tumor can take up to 40 years to appear. Projections from the end of the last century suggested that the incidence of MPM in Western Europe would peak around 2020[6], and recent epidemiological data would appear to confirm this trend. MPM incidence rates are lower in some parts of Asia and Central and Eastern Europe, but this could be due to less rigorous data collection or reporting[7] or higher mortality due to other causes. In short, based on the available data, MPM incidence and mortality rates vary considerably according to geographic location.
Asbestos: Asbestos is the main cause of MPM. This mineral was widely used for many years in construction products (e.g., roofing and tiles), friction materials, packaging, textiles, paint, and a range of other industrial products.
Although the association between asbestos exposure and MPM has been demonstrated[8], it has not been possible to define cumulative exposure limits, meaning that anyone exposed to asbestos is potentially at risk.
Occupational exposure accounts for over 80% of MPM cases in men[9-12], explaining differences in attributable risk between men and women.
Other minerals: Other elongated mineral particles such as erionite[13,14] and fluoro-edenite[15] may also have a causative role in MPM. Environmental exposure to these minerals is higher in certain countries, such as Turkey, the United States, and Mexico[16-18].
Genetic predisposition: Familial aggregation studies have also reported an increased risk of MPM among offspring and siblings of patients with the disease[19-21]. This increased risk has been linked to a germline mutation in BAP-1 (breast cancer gene 1-associated protein), which is a tumor suppressor gene with a role in DNA transcription and repair[22,23].
Germline mutations in cancer-susceptibility genes have been reported in a significant proportion of MPM patients, particularly those with peritoneal mesothelioma, younger patients, patients with minimal asbestos exposure, and those with a second cancer[24,25].
The treatment options for MPM are largely determined by stage, and approaches vary depending on whether the patient has operable or inoperable disease and is deemed suitable for surgery. Surgery, radiotherapy, and systemic therapies are constantly evolving to meet better the challenges associated with each stage of disease.
Hemithoracic radiotherapy as an adjunct to pneumonectomy has been used in MPM for years, but its effect on disease control remains modest. As a palliative measure, it has proven effective at improving pain and quality of life.
More advanced radiotherapy techniques [respiratory control, four-dimensional imaging, intensity-modulated radiotherapy (IMRT), stereotactic body radiation therapy (SBRT), and proton therapy] have since emerged and are used in combination with induction chemotherapy and/or surgery or as radical therapy in patients with unresectable disease.
Radiotherapy has also been proposed as a salvage strategy for patients whose disease recurs following surgery or chemotherapy. Its role for improving progression-free and overall survival (OS) in patients with oligoprogressive disease is also being investigated.
As mentioned, no optimal treatment strategies have yet been established for MPM, and recent clinical guidelines offer contrasting conclusions and recommendations, even though they are based on the same scientific evidence. These guidelines include the British Thoracic Society Guideline for the Investigation and Management of Malignant Pleural Mesothelioma[26], the National Comprehensive Cancer Network Malignant Pleural Mesothelioma Guidelines[27], the American Society of Clinical Oncology Clinical Practice Guideline for the Treatment of Malignant Pleural Mesothelioma[28], and the European Respiratory Society/European Society of Thoracic Surgeons/European Association for Cardio-Thoracic Surgery/European Society for Radiotherapy and Oncology Guidelines for the Management of Malignant Pleural Mesothelioma[29].
In the current clinical practice guidelines, we will apply the system used by the European Society of Medical Oncology[30] to guide decision-taking regarding the management of MPM (which in turn was adapted from the Infectious Diseases Society of America’s grading system for ranking recommendations in clinical guidelines[31]). Accordingly, the strength of a given recommendation for or against a preventive or therapeutic practice is graded using the letters A-E, while the quality of the supporting evidence is graded using the Roman numerals I-III (Table 1).
| IDSA United States Public Health Service Grading System for Ranking Recommendations in Clinical Guidelines | ESMO adaptation of IDSA Grading System | ||
| Category, grade | Definition | Category, grade | Definition |
| Strength of recommendation | Grades of recommendation | ||
| A | Good evidence to support recommendation for use | A | Strong evidence for efficacy with a substantial clinical benefit, strongly recommended, strongly recommended |
| B | Moderate evidence to support recommendation for use | B | Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended |
| C | Poor evidence to support a recommendation | C | Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs, etc.), optional |
| D | Moderate evidence to support a recommendation against use | D | Moderate evidence against efficacy or for adverse outcome, generally not recommended |
| E | Good evidence to support a recommendation against use | E | Strong evidence against efficacy or for adverse outcome, never recommended |
| Quality of evidence | Levels of evidence | ||
| I | Evidence from > 1 properly randomized, controlled trial | I | Evidence from at least one large randomized, controlled trial of good methodological quality (low potential for bias) or meta-analyses of well-conducted randomized trials without heterogeneity |
| II | Evidence from > 1 well-designed clinical trial, without randomization; from cohort or case-controlled analytic studies (preferably from > 1 center); from multiple time series; or from dramatic results from uncontrolled experiments | II | Small randomized trials or large randomized trials with a suspicion of bias (lower methodological quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity |
| III | Evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees | III | Prospective cohort studies |
| IV | Retrospective cohort studies or case-control studies | ||
| V | Studies without control group, case reports, experts opinions | ||
The grades of recommendation and levels of evidence for the use of surgery, chemotherapy, and radiotherapy in MPM are summarized in Table 2.
| Surgery | Chemotherapy | Radiotherapy |
| For palliation of pleural effusions when patients cannot benefit from chest tube drainage or chemical pleurodesis or when these are not successful (II, A) | The anti-folate/platinum doublet is the only approved standard of care for the first- and second-line treatment of unresectable mesothelioma (I, A); If available, bevacizumab, could be added to the standard treatment in selected patients (II, B) | For palliation of pain related to tumor growth radiotherapy can be considered (II, A) |
| To obtain diagnostic samples of tumor tissue and to stage the patient (II, A) | Maintenance therapy (switch or continuation) has not yet improved overall survival and patients should be included in these studies (II, A) | The use of radiotherapy to prevent growth in drainage tracts is not proved to be useful (III, A) |
| To be part of a multimodality treatment, preferably as part of a study (II, A) | Patients in good condition should be recommended to join studies in second line (II, A) | Radiotherapy can be given in an adjuvant setting after surgery or chemo-surgery to reduce the local failure rate. However, no evidence is available for its use as a standard treatment (II, A) |
| To perform a macroscopic complete resection by means of pleurectomy/decortication (III, C) | When postoperative radiotherapy is applied, strict constraints must be adhered to in order to avoid toxicity to neighboring organs, and special, tissue sparing, techniques should be used (II, A) |
Chest radiography normally shows pleural effusion and thickening.
Computed tomography (CT) of the chest is the first-line imaging test for patients with suspected MPM. It typically shows findings suggestive of MPM, such as thickening of the mediastinal pleura[32].
Positron emission tomography (PET)-CT may be useful in certain cases but should not be used in patients who have undergone pleurodesis as this procedure can affect maximum standardized uptake values. PET-CT has low sensitivity for stage N1 (38%) and T4 (76%) disease[33]. Compared with CT alone, it has higher specificity for disease stages II (100% vs 77%) and III (100% vs 75%)[34].
Magnetic resonance imaging (MRI) has not yet been validated in MPM. It may be useful for identifying margins in patients with multifocal chest wall involvement and for demonstrating unresectability.
Thoracoscopy is the diagnostic technique of choice in MPM. Other less invasive biopsy techniques, however, can be used in certain cases.
Closed pleural biopsy: Closed (blind) pleural biopsy with an Abrams needle has a diagnostic sensitivity of 27%-60% for malignancy[35,36]. Complications are common, in particular pain and pneumothorax (around 15%). In the largest review to date of closed pleural biopsies (n = 2893), the diagnostic yield for malignancy was 57%[37].
Image-guided pleural biopsy: A number of studies have reported higher diagnostic sensitivity (around 88%[38]) for image-guided cutting-needle biopsy compared with closed pleural biopsy.
Maskell et al[39] demonstrated that CT-guided cutting-needle biopsy was approximately 40% more sensitive than Abrams biopsy for diagnosing malignancy. The technique correctly diagnosed malignancy in 13 of 15 patients with suspected malignant pleural effusions (sensitivity, 87%; specificity, 100%).
In 1999, Heilo et al[40] reported that ultrasound-guided core-needle biopsy had a diagnostic sensitivity of 77%, a specificity of 88%, and a positive predictive value of 100% for MPM.
Thoracoscopy: Thoracoscopy has a high diagnostic yield in pleural malignancies. In an analysis of 1369 patients from 22 case series, thoracoscopy had a diagnostic sensitivity of 92.6% for malignant pleural disease. Complication rates are very low, and a mortality rate of 0.34% has been reported[41]. Thoracoscopy has been found to have superior diagnostic sensitivity and specificity than both closed and image-guided pleural biopsy[42,43].
Video-assisted thoracoscopic surgery: Numerous case series have found video-assisted thoracoscopic surgery (VATS) to have high diagnostic sensitivity and specificity for malignant pleural disease[44,45].
Although no direct comparisons have been made of medical and surgical thoracoscopy, data suggest that the two procedures have a very similar sensitivity.
VATS pleural biopsy has a sensitivity of 95%, a specificity of 100%, and a negative predictive value of 94%[44,45].
MPM should be diagnosed according to the 2015 World Health Organization (WHO) Classification using an adequate tissue sample.
MPM can be divided into diffuse malignant mesothelioma (DMM), localized malignant mesothelioma (LMM), and well-differentiated papillary mesothelioma (WDPM). DMM has a worse prognosis than LMM and WDPM.
DMM can be further categorized as epithelioid, sarcomatoid, or biphasic. The second two subtypes are associated with worse survival[46]. Pleomorphic epithelioid DMM is a particularly aggressive variant[47].
Cytologic examination of pleural fluid is usually the first diagnostic test, as pleural effusion is typically the first clinical sign detected. Not all epithelioid subtypes, however, cause pleural effusion, and sarcomatoid subtypes do not generally spread to the serosal cavity.
Cytology may also be useful when pleural biopsy is not possible.
The 2015 WHO classification of tumors of the lung, pleural, thymus, and heart introduced a number of changes in relation to MPM. First, it recognized that the pleomorphic subtype, like the sarcomatoid variant, is associated with poor prognosis. It also recognized the usefulness of immunohistochemistry for distinguishing between MPM and carcinoma, and redefined the criteria for differentiating MPM from reactive mesothelioma proliferations.
The discovery that the NAB2-STAT6 fusion was a hallmark of solitary fibrous tumors had important diagnostic implications. The WWTR1-CAMTA1 fusion is a marker of epithelioid hemangioendothelioma, while desmoid-type fibromatosis is frequently associated with CTNNB1 mutations and beta-catenin expression.
Immunohistochemistry is an important diagnostic tool as it can help distinguish between epithelioid mesothelioma and other carcinomas involving the pleura (mainly lung adenocarcinoma).
Claudin 4 has emerged as one of the most useful markers for differentiating mesothelioma (claudin 4-) from adenocarcinomas (claudin 4+)[48].
Calretinin, cytokeratin 5/6, Wilms tumor 1, and D2-40 are all markers of mesothelioma, while carcinoembryonic antigen, B72.3, Bg8, BerEP4, and M031 are markers of carcinoma[49].
Epithelioid DMM can sometimes resemble squamous cell carcinoma, but it does not show nuclear staining with p40 (or p63)[50].
The new WHO Classification also recognizes the difference between DMM and reactive mesothelial proliferations or hyperplasia. Given the absence of specific markers for benign mesothelial processes, the distinction between benign and malignant proliferations is usually based on morphologic features.
WDPM, can be difficult to distinguish from conventional forms of DMM. It is characterized by small translucent nodules, whereas DMM usually shows solid nodules and pleural effusion or thickening. It also follows a characteristically indolent course[51].
The three best-defined genetic alterations in MPM are loss of neurofibromatosis type 2 (45%-50% of cases), homozygotic deletion of CDKN2A (p16) (100% of sarcomatoid cases), and loss of BAP-1 (45%-100% of cases, most of which are epithelioid). BAP-1 loss is associated with good prognosis, while CDKN2A loss is associated with poor prognosis.
Eighth edition of the TNM classification for mesothelioma (Tables 3 and 4)
| T | Primary tumor | N | Regional lymph nodes |
| Tx | Primary tumor cannot be assessed | Nx | Regional lymph nodes cannot be assessed |
| T0 | No evidence of primary tumor | N0 | No regional lymphnodemetastases |
| T1 | Tumor limited to the ipsilateral parietal pleura with or without involvement of visceral pleuralmediastinal pleuradiaphragmatic pleura | N1 | Metastases in the ipsilateral bronchopulmonary, hilar or mediastinal (including the internal mammary, peridiaphragmatic, pericardial fat pad or intercostal lymph nodes) lymph node) |
| T2 | Tumor involving each of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic and visceral pleura) with at least one of the following features: Involvement of diaphragmatic muscle. Extension of tumor from visceral pleura into the underlying pulmonary parenchyma | N2 | Metastases in the contralateral mediastinal, ipsilateral or contralateral supraclavicular lymph nodes |
| T3 | Locally advanced but potentially resectable tumor. Tumor involving all of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic, visceral pleura) with at least one of the following features: Involvement of endothoracic fascia; Extension into the mediastinal fat; Non-transmural involvement of the pericardium; Solitary, completely resectable focus of Tumor extending into the soft tissues of the chest wall | M | Distant metastasis |
| T4 | Locally advanced technically unresectable tumor. Tumor involving all of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic and visceral pleura) with at least one of the following features: Diffuse extension or multifocal masses of tumor in the chest wall, with or without associated rib destruction; Direct transdiaphragmatic extension of tumor to peritoneum; Direct extension of tumor to the contralateral pleura; Direct extension of tumor to mediastinal organs; Tumor extending through to the internal surface of the pericardium with or without pericardial effusion, or tumor involving the myocardium | M0 | No distant metastasis |
| M1 | Distant metastasis present |
| T | N | M | |
| Stage IA | T1 | N0 | M0 |
| Stage IB | T2-T3 | N0 | M0 |
| Stage II | T1-T2 | N1 | M0 |
| Stage IIIA | T3 | N1 | M0 |
| Stage IIIB | T1-T3 | N2 | M0 |
| T4 | Any N | ||
| Stage IV | Any T | Any N | M1 |
The new TNM staging system for mesothelioma is summarized in Tables 3 and 4. The tables were created by the International Association for the Study of Lung Cancer (IASLC) and are published in the eighth edition of the American Joint Committee on Cancer Staging Manual[52-54].
Only about 20% of patients with MPM are candidates for potentially curative treatment.
Surgery is the first-line option for localized disease. The combined use of surgery, chemotherapy, and radiotherapy has not been established as first-line treatment due to conflicting data from the main studies conducted to date.
Radiotherapy is mainly used as adjuvant or neoadjuvant therapy in MPM. Radiotherapy with curative intent has limited applications in unresectable disease because of the challenges associated with treating large volumes with doses of > 60 Gy without damaging adjacent organs[55,56]. Radiotherapy alone is therefore mostly used for palliative purposes in MPM.
Surgery for staging and palliative purposes: The MesoVATS randomized clinical trial, which compared VAT-partial pleurectomy and standard talc pleurodesis in 196 patients, found no significant differences in OS between the groups[57]. VAT-partial pleurectomy, however, was associated with better pleural effusion control and quality of life at 6 and 12 mo[57].
Surgery with radical intent-trimodality therapy: The goal of surgery with radical intent in MPM is to achieve macroscopic resection with maximal cytoreduction. Techniques include extrapleural pneumonectomy (EPP), pleurectomy/decortication (P/D), and extended P/D. Recommendations for the uniform definitions of these techniques were established by the IASLC Staging Committee and the International Mesothelioma Interest Group[58].
Trimodality therapy, which is the combination of chemotherapy, surgery, and radiotherapy, can increase survival in patients with resectable MPM. Nonetheless, over 50% of patients are unable to complete treatment due to toxicity and/or previous comorbidities (American Society of Anesthesiologists score > 3, forced expiratory volume in 1 s < 70%, and/or smoking)[59].
Multivariate analyses of retrospective data have shown that EPP is associated with slightly higher operative mortality and lower OS than P/D. Such comparisons, however, are prone to bias as choice of technique by a given institution depends on its experience[60,61]. Until further clinical evidence becomes available, both EPP and P/D can be considered for surgery with radical intent. Choice of technique should be decided by an expert committee on a case-by-case basis.
Studies of curative-intent trimodality therapy have evaluated different regimens. Induction chemotherapy has been shown to increase the rate of complete resections in early-stage MPM.
A multicenter Swiss trial that analyzed the effects of radiotherapy after neoadjuvant chemotherapy and EPP in patients with resectable MPM reported an encouraging median survival of 23 mo[62].
The European Organisation for Research and Treatment of Cancer investigated the feasibility of trimodality therapy with EPP in a phase II trial (European Organisation for Research and Treatment of Cancer 08031)[63]. The primary endpoint (treatment success defined as a patient who received the full treatment and was still alive 90 d after the end of treatment without progression or grade 3-4 toxicity) was not achieved. Another phase II trial investigating trimodality therapy in MPM that recruited 77 patients from nine institutions in the United States reported an operative mortality rate of 7% and a median OS of 16.8 mo[64].
Although trimodality therapy seemed feasible in selected patients[65], the Mesothelioma and Radical Surgery 1 randomized trial was designed to compare induction chemotherapy alone with induction chemotherapy plus EPP. It did not, however, achieve the planned sample size. Just 50 patients (45% of those recruited) were randomly assigned to either EPP or no EPP after chemotherapy, and just 16 of those in the EPP arm completed treatment. The mortality rate in this group was 18.8%[66]. The results of the subsequent trial, Mesothelioma and Radical Surgery 2, evaluating chemotherapy alone and chemotherapy followed by P/D are eagerly awaited.
The authors of a systematic review concluded that trimodality therapy can benefit selected patients in experienced centers[67].
One of the most important papers on trimodal therapy is the phase III trial by Trovo et al[68] published in 2020. In this trial, 108 patients with non-metastatic MPM, after non radical lung-sparing surgery and chemotherapy, were randomly assigned to receive hemithoracic radiation therapy (50 Gy in 25 fractions, gross residual disease received a boost of 60 Gy) or palliative radiation therapy (doses between 20-30 Gy). The primary endpoint was OS. With a median follow-up of 14.6 mo, the 2-year OS rate was 58% in the hemithoracic radiation therapy arm vs 28% in the palliative radiation therapy arm (hazard ratio, 0.54). However, in the hemithoracic radiation therapy group, the acute toxicity grade ≥ 3 was 20%, and the late toxicity grade ≥ 3-4 was registered in 17 patients (31% of the initial sample).
Adjuvant and neoadjuvant radiotherapy: Relatively little has been published on the use of adjuvant and neoadjuvant radiotherapy in MPM.
The Surgery for Mesothelioma after Radiation Therapy trial is a phase I-II trial that analyzed the feasibility of a short course of neoadjuvant hemithoracic IMRT (25 Gy in five fractions over consecutive days with a concomitant 5 Gy boost to risk areas) followed by EPP in patients with resectable T1-3N0M0 MPM[69]. The results were promising, with a cumulative 3-year survival rate of 84% in patients with epithelioid subtypes.
In 2016, de Perrot et al[70] published the results of a study of 62 patients treated with the Surgery for Mesothelioma after Radiation Therapy protocol. They reported a median survival of 36 mo. Respective median OS and disease-free survival (DFS) were 51 and 47 mo for patients with epithelioid-subtype tumors vs 10 and 8 mo for those with biphasic subtypes (P = 0.001).
Based on the results of the above two studies, neoadjuvant radiotherapy could be considered for patients with potentially resectable (T1-3N0M0) epithelioid subtype. Further randomized trials are needed.
The earliest studies of adjuvant radiotherapy in MPM reported high rates of serious adverse events. Pulmonary toxicity was particularly common after decortication.
The SAKK trial randomized 54 patients to observation or adjuvant radiotherapy after EPP but was terminated prematurely due to insufficient recruitment[71].
Nelson et al[72], using data from the United States National Cancer Database, analyzed the role of adjuvant radiotherapy in MPM patients treated between 2004 and 2013. Of the 2846 patients who underwent surgery, 213 (7%) received adjuvant radiotherapy, which was associated with better survival in patients with stage I-II disease (P = 0.024) but not in those with stage III (P = 0.890) or IV (P = 0.183) disease.
Prophylactic radiotherapy: The role of prophylactic radiotherapy in MPM is controversial, as the results of more recent randomized clinical trials contradict findings from older studies and small trials from the pre-chemotherapy era.
Two randomized trials comparing immediate radiotherapy to the tract (21 Gy/3 fractions) and observation only concluded that radiotherapy did not reduce the incidence of tumor seeding[73,74]. Similarly, the trial by Clive et al[75] found no differences between immediate and deferred prophylactic radiotherapy, contrasting with findings by Boutin et al[76] in 1995 indicating that early radiotherapy prevented malignant seeding.
Prophylactic radiotherapy is not recommended in clinical practice. Where possible, patients should be included in clinical trials such as prophylactic irradiation of tracts (NCT01604005).
Chemotherapy is the treatment of choice for unresectable locoregional or metastatic MPM. It has been demonstrated to improve both survival and quality of life[77]. Nonetheless, palliative care is the only option in certain patients due to age, comorbidities, or poor general health.
The main goal of palliative radiotherapy is to alleviate pain caused by tumor invasion of the thoracic structures[78].
The evidence supporting radiotherapy to the chest for pain relief is based on a systematic review of retrospective series and small phase II trials[79]. The authors, however, were unable to draw any useful conclusions about the benefits of palliative radiotherapy in MPM or about optimal dose fractionation or target volumes due to the use of highly heterogeneous protocols and the fact that most of the techniques employed are now obsolete. The best evidence to date is from the multicenter phase II SYSTEMS-1 trial, which used modern radiotherapy techniques[80]. The trial included 30 patients who received 20 Gy (five fractions of 4 Gy/d). Pain, treatment response, and quality of life were evaluated using standardized questionnaires at weeks 1, 5, and 12 post-treatment. The results showed an improvement in pain in 47% of patients. No significant effects were observed for the other quality-of-life variables.
The encouraging results of SYSTEMS-1 led the researchers to design a second trial, SYSTEMS-2[81], to compare the effects of an escalated dose (36 Gy in six fractions for 2 wk) and a standard dose (20 Gy in five fractions for 1 wk) delivered only to the tumor responsible for the pain (hemithoracic radiation was not contemplated). The planned sample size is 112 patients and patients will be treated with IMRT or, where not available, three-dimensional (3D) radiotherapy. Radiotherapy quality, treatment responses, and associated toxicity will be evaluated using predefined protocols. No results have been reported yet.
First-line: The goal of first-line systemic therapy is to improve survival in patients with unresectable MPM. Superior survival outcomes have been reported for cisplatin plus pemetrexed or raltitrexed compared with cisplatin alone in phase III trials[77,82]. Carboplatin is an acceptable alternative, especially in older patients[83,84]. A number of clinical trials have investigated the effects of adding other agents to standard treatment. The phase III MAPS trial[85] showed the benefits of adding bevacizumab to the cisplatin-pemetrexed doublet, but this protocol has not been incorporated into general practice as it did not receive authorization from the United States Food and Drug Administration or the European Medicines Agency.
Maintenance: Continuation and switch maintenance chemotherapy with pemetrexed have changed clinical practice for non-small cell lung cancer. Their efficacy has not yet been demonstrated in MPM, but it is being investigated in the ongoing COMMAND and NVALT-19 trials.
Second-line: There is no current second-line standard-of-care systemic therapy for MPM, although a trend towards improved survival has been observed for vinorelbine[86,87], supporting findings for this drug as first-line therapy in the MSO1 trial[88].
Immunotherapy: The effect of tremelimumab on survival in patients with unresectable MPM is being evaluated in a phase III randomized trial (NCT01843374)[89]. PDL1 has also emerged as a promising target for immunotherapy, as it is significantly expressed by MPM and sarcomatoid mesothelioma in particular. In the absence of second-or further-line standard of care, patients should be encouraged to enroll in clinical trials where possible.
Defining target volumes for radiotherapy in MPM is challenging and requires thorough knowledge of the anatomy of the thorax and diaphragm and at times close collaboration with the thoracic surgeon.
Candidates for radiotherapy should have adequate performance status [electrocorticogram (ECOG) score ≤ 1] and respiratory function (forced expiratory volume in 1 s > 80%).
Patients should be placed in a supine position with their arms raised over their head using a patient-specific immobilization device, such as an alpha cradle and headrest. Radiopaque fiducial markers and/or boluses should be placed in the region of the surgical incision and drainage areas.
CT images with a slice thickness of 2.5-3 mm should be acquired from 5 mm above the lung apex to the lower margin of the L3 vertebra or the anterior superior iliac spine, to include both kidneys.
Four-dimensional CT scans are recommended to determine the position of the diaphragm during respiratory movements. PET-CT may be necessary for postoperative restaging, particularly in patients with positive margins.
In patients with residual macroscopic disease, MRI with T1-and T2-weighted sequences, fat suppression, and diffusion-weighted imaging can be useful for determining macroscopic tumor volume[90]. Accuracy of target volume delineation can be improved by the intraoperative placement of fiducial markers at the level of the lower insertion of the diaphragm (or reconstructed diaphragm in cases of resection) and the anterior medial pleural reflection. It is also helpful to place these markers in any areas where the thoracic surgeon deems that complete resection may not be possible.
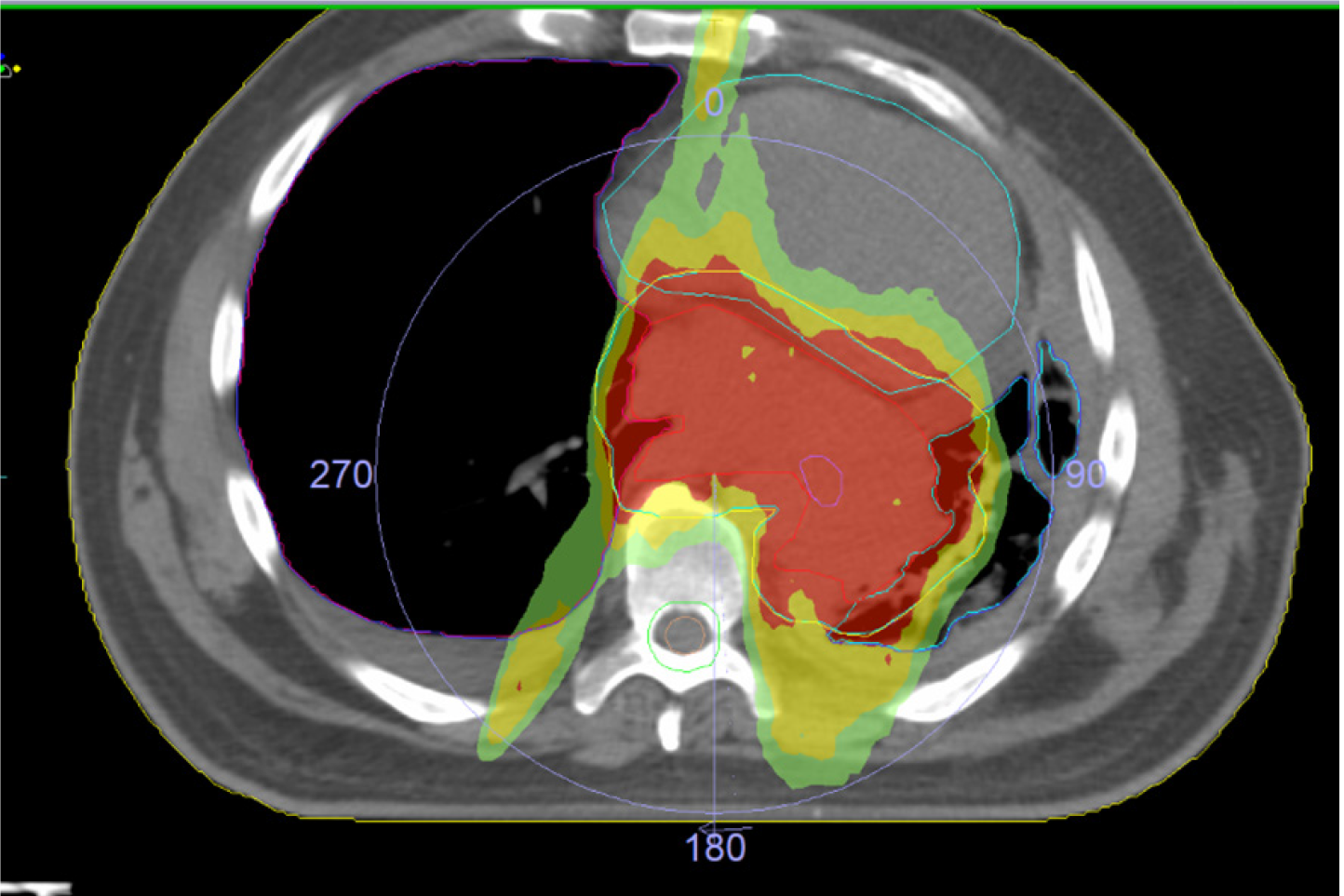
Clinical target volume (CTV): Volume encompassing entire pleura and ipsilateral chest wall as well as any sites at risk of residual disease (Figure 1).
Superior border: Above the first rib or 5 mm above the superior chest incision, whichever is higher.
Inferior border: Should encompass the entire diaphragmatic dome and diaphragmatic insertion as far as the emergence of the psoas muscle.
Anterior, posterior, and lateral borders: Should encompass the full thickness of the chest wall, ribs, intercostal muscles, lateral border of the sternum, costovertebral joints, lateral border of the vertebral bodies, costomediastinal and costodiaphragmatic recesses, and diaphragmatic crus. Anteromedially: Should encompass the anterior pericardium and the anterior medial pleural reflection. A 5-mm expansion margin should be used on the outer part of the chest wall, including skin in surgical incision and drainage areas. A 1-mm expansion margin should be used on the inner part of the chest wall in patients who have undergone lung-sparing surgery, bearing in mind that an additional 5 mm will be added for the planning target volume (PTV).
Medial border: Should encompass the mediastinal pleura and ipsilateral hilum. The inclusion of mediastinal lymph nodes is controversial. The proportion of positive mediastinal nodes ranges from 35%–41% depending on the surgical series[91,92], hence the recommendation by some groups to include these nodes in the radiation field[91,93-95].Although this positivity rate is high, most recurrences observed in cases where only involved mediastinal nodes were included occurred in the contralateral hemithorax (38%) or the abdomen (33%). Just 5% of local recurrences were classified as in-field failures[92]. Most groups therefore recommend only including mediastinal lymph nodes if they are positive[63,70,96-100].
PTV: Generally corresponds to the CTV with an isotropic expansion margin of 5 mm. It may, however, vary depending on the immobilization and/or image-control techniques used.
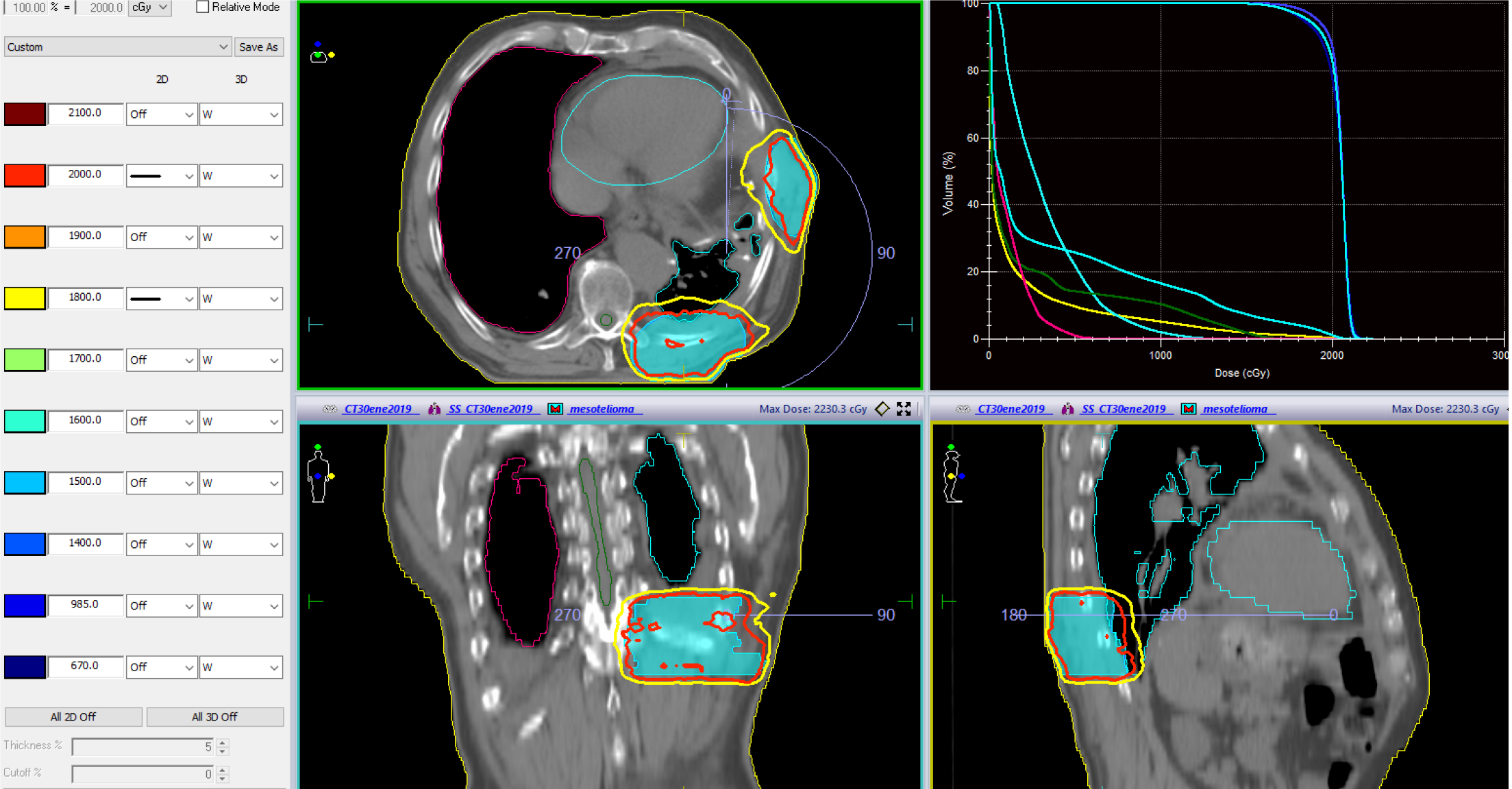
The recommended radiation dose is 50–54 Gy in daily fractions of 1.8–2 Gy. Patients with residual disease will require a boost to at least 60 Gy. Organs at risk (OAR) dose constraints must be met at all times (Table 5).
| Organs at risk | Dose constraints |
| Lungs-GTV | V20 < 37%, Mean dose < 20 Gy |
| Contralateral lung-PTV | V20 < 20%, V5 < 17%, Mean dose < 8 Gy |
| Ipsilateral lung | V40 < 67%, Mean dose < 36 Gy |
| Heart defined as pericardial sac | Right mesothelioma V40 < 25%; Left mesothelioma V40 < 35% |
| Brachial plexus | Maximum dose < 65 Gy |
| Esophagus | V55 Gy < 30%; Mean dose < 34 Gy |
| Stomach minus including PTV | Mean dose < 30 Gy |
| Bowel | Maximum dose < maximum PTV < 55 Gy; D5 cc < 50 Gy |
| Spinal cord | Maximum dose < 50 Gy |
| Liver minus GTV | V30 < 45%; Mean dose < 30 Gy |
| Kidneys evaluated separately | V18 < 33% (or V18 < 50%, if cannot be achieved at ≤ 33%) |
| Ipsilateral kidney; Contralateral kidney | V25 < 40%; V10 < 10% |
OARs must be contoured according to the radiation therapy oncology group atlas[101] (Table 6).
| Structure | Heart | Contralateral lung | Ipsilateral lung | Esophagus | Spinal cord |
| Dose constraints | V40: 0 (< 35%) | V20: 1.5 (< 20%); Mean dose 7 Gy (< 8) | V40: 57 (< 67%); Mean dose: 35 Gy (< 36 Gy) | V55: 0 (< 30%); Mean dose 26 (< 34 Gy) | Maximum dose: 43.7 (< 50 Gy) |
IMRT techniques are recommended for treatment planning. The PTV must be covered by at least 95% of the prescribed dose (Figure 2).
Choice of radiotherapy technique depends on the clinical context, treatment goal (curative or palliative intent), planned dose, and location of the target volume and OARs.
Conventional two-dimensional or 3D techniques are suitable for most treatments with palliative intent. The doses are normally low (20 Gy in five fractions or 30 Gy in 10 fractions), and long-term toxicity is less of a concern given the poor prognosis[80].
Higher doses are needed when the goal is to achieve good long-term local control. More highly conformal techniques are recommended to achieve this goal with as little toxicity as possible.
Many studies of radiotherapy in MPM have used conventional postoperative two-dimensional hemothoracic radiotherapy with parallel opposing photon fields, with or without an electron boost. This technique results in good sparing of the contralateral lung but is associated with significant treatment inhomogeneities linked to a high risk of local failure and/or toxicity[102]. Technological advances brought with them a shift towards an increasing use of 3D conformal radiotherapy techniques, which enable improved definition of target volumes. Even more advanced techniques emerged, offering superior conformality, precision, image-guided control, and dose distribution [IMRT, volumetric modulated arc therapy (VMAT), helical tomotherapy (HT), and proton therapy[103]].
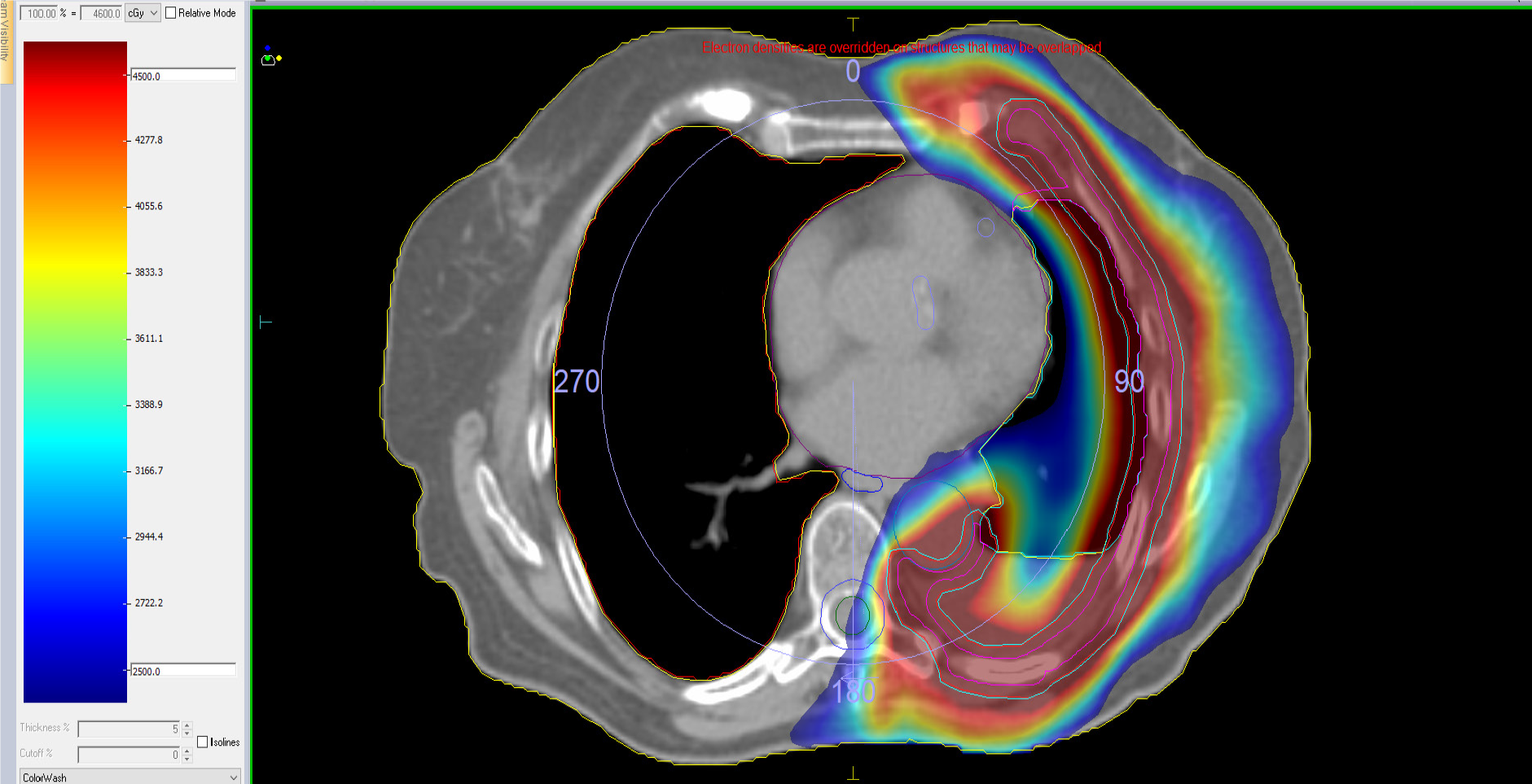
A typical clinical case of modern postoperative RT after P/D is showed in Appendix 1.
IMRT and image-guided radiotherapy (IGRT) allow for the use of more highly conformal treatments, enabling thus the delivery of potentially curative doses to complex target volumes while maintaining doses to surrounding healthy tissue at an acceptable level[104-106].
The potential of IMRT as adjuvant therapy has been evaluated in several trials, the earliest of which demonstrated the feasibility of hemithoracic IMRT following EPP. Institutional studies of modern radiotherapy techniques have reported 2-year locoregional control rates ranging from 40%–71% and 2-year OS rates of 18%–57%[95,107-110].
In a study conducted at the MD Anderson Cancer Center (MDACC), EPP followed by IMRT was associated with a locoregional failure rate of just 13%[111]. Buduhan et al[112] reported that IMRT was associated with lower local recurrence rates than conventional radiotherapy in patients treated with induction chemotherapy and EPP.
Better local control rates were also observed in another two prospective phase II trials. In the first, Krug et al[64], studied a series of patients treated with EPP and adjuvant IMRT (54 Gy in 30 fractions) and reported a local failure rate of 20.4% and a median survival of 29.1 mo. In the second phase II trial, Van Schil et al[63] reported a locoregional recurrence rate of just 16.2% and a median survival of 33 mo in patients treated with trimodality therapy comprising postoperative radiotherapy at a dose of 54 Gy in 30 fractions.
Despite the excellent results reported in a number of studies[113], IMRT following EPP has been associated with severe pulmonary toxicity[56,111-114]. Researchers at the Dana-Faber Cancer Institute also reported high mortality rates attributed to IMRT[115]. The patients had been treated with IMRT after EPP and adjuvant chemotherapy and had received a dose of 54 Gy and a boost up to 60 Gy in high-risk areas (positive margins or areas with residual disease). Six of the 13 patients (46%) died after receiving IMRT. In a retrospective review of 63 patients treated with either 3D radiotherapy or IMRT, Rice et al[56] found a 6-mo mortality rate of 37% (23 patients). Of these 23 patients, six died of a fatal pulmonary event. Five (5.8%) of the patients in a study performed by Gómez et al[109] at the MDACC experienced grade 5 pulmonary toxicity.
Severe toxicity in the above studies was associated with neoadjuvant chemotherapy and several dosimetric parameters, namely V20 (contralateral lung volume receiving 20 Gy), V5, V10, and mean lung dose[111,115]. The recommendation thus was to restrict these doses for OARs.
The higher number of radiation beams or fields used in treatments plans for IMRT, VMAT, and HT means that the volume of tissue receiving low doses (5–10 Gy) may be larger, and this could directly affect toxicity. It is important to keep the above dosimetric parameters as low as possible for the contralateral lung. No specific thresholds have been established, but patients who experienced severe pulmonary toxicity in the above studies had mean lung dose > 8.5 Gy, V5 > 80%, V10 > 55% and V20 > 7%. The current recommendation thus is not to exceed these levels for any of the four parameters. V20 was the only independent dosimetric predictor of grade 5 pneumonitis or disease-specific death[111]. Dose constraints to the lung have become increasingly strict as our understanding of IMRT has improved. The result has been a reduction in the incidence of grade 3 or higher pneumonitis (< 10% in most series) and low rates of grade 3 or higher esophagitis, asthenia, and dermatitis[116,117].
Patel et al[117] found that greater experience with IMRT planning in patients with MPM following EPP was associated with statistically significant better coverage of target volumes and a significant decrease in the dose to the contralateral lung, with relatively low lung toxicity rates (13%).
Researchers from the University Hospital of Heidelberg analyzed IMRT delivered using the step-and-shoot technique or HT[110]. The median dose administered was 48–54 Gy, and there were no grade 4 or higher toxicities. Median OS was 20.4 mo. SAKK 17/04, an international multicenter phase II randomized trial, is the largest trial conducted to date of patients with MPM and the first to evaluate the role of high-dose hemithoracic radiotherapy as part of a multimodality approach. Following treatment with neoadjuvant chemotherapy (cisplatin/pemetrexed) and EPP, patients were randomized to receive or not high-dose radiotherapy (45.5 Gy in 26 fractions and a simultaneous integrated boost up to 55.9 Gy) to the hemithorax, the thoracotomy scar, and the mediastinal lymph node stations. The trial was terminated early due to low accrual. The difference in locoregional recurrence-free survival between patients in the high-dose radiotherapy group and the non-radiotherapy group was not significant (9.4 vs 7.6 mo)[70].
In conclusion, despite the results of the SAKK 17/04 trial, increasing experience with IMRT delivery and planning has led to improved toxicity profiles and lower local recurrence rates (15%–35%)[109,110]. Hemothoracic radiotherapy continues thus to be the recommended standard of care following EPP for patients with operable stage I to III MPM. This indication features in several recent guidelines[27-29,118], which also recommend that this technique be performed in centers of excellence with experience in this modality.
There is an increasing trend to treat operable MPM using more conservative, less invasive surgical techniques, such as P/D, which is associated with lower morbidity and mortality than EPP. P/D, however, may be less effective at achieving adequate cytoreduction and carries a higher risk of local and distant recurrence. The use of adjuvant radiotherapy thus must be contemplated in such cases. The presence of an intact ipsilateral lung in addition to other OARs after P/D also poses a major challenge to radiation oncologists[28].
The earliest studies of conformal radiotherapy following P/D showed a high incidence of grade 3 or higher toxicities (mostly pulmonary) and low survival rates[107,119].
Gupta et al[119] reviewed the records of 123 patients from the Memorial Sloan-Kettering Cancer Center treated with P/D followed by hemithoracic radiotherapy (3D with or without brachytherapy) over a 30-year period. With a mean follow-up time of 11 mo, median survival was 13.5 mo. Actuarial OS at 1 year was 23% and the local recurrence rate was 56.1%. The authors concluded that the combination of P/D and adjuvant hemithoracic radiotherapy was not effective and advised against the use of conventional IMRT in this setting[119].
Local failure rates following the clinical implementation of IMRT after P/D vary considerably from one study to the next, with 2-year rates ranging from 40%–68%; there have also been reports of severe toxicities[28].
According to a recent systematic review of the safety and efficacy of IMRT following P/D by Patel et al[120], between 0% and 16% of patients develop grade 3 pneumonitis, while less than 1.5% develop grade 4 or 5 pneumonitis. The authors also reported a recurrence rate of 19%–60%, a mean DFS time of 12–16 mo, and OS of 19–28 mo.
The Memorial Sloan-Kettering Cancer Center has published some of the most significant findings for IMRT as adjuvant therapy for MPM patients treated with P/D. Rosenzweig et al[121] reviewed the results of 36 patients treated with hemithoracic IMRT at this center; 20 had undergone P/D, and 16 had biopsy only. The dose in all cases was 50.4 Gy (1.8 Gy/fraction). The authors reported a grade 3 or higher toxicity rate of ≥ 20% and a median survival of 26 mo. They concluded that adjuvant IMRT following P/D improved disease-free and OS with acceptable toxicity levels[121]. The same group analyzed data from an additional 67 patients treated with IMRT (28 after P/D)[122]. The respective 1- and 2-year actuarial failure rates were 28% and 40%, and most of the recurrences were distant.
The first prospective phase II study to evaluate the safety of adjuvant hemithoracic IMRT after chemotherapy and P/D concluded that the administration of 50.4 Gy in 28 sessions was feasible[100]. There were no cases of grade 4 or 5 pneumonitis; 29.6% of patients developed radiation pneumonitis, and the median disease-free and OS times were 12.4 and 23.7 mo, respectively. The disease recurred locally in 58% of patients. Most of these recurrences were at sites with prior macroscopic disease, highlighting the importance of complete macroscopic resection.
Shaikhet al[123] retrospectively reviewed the data of 209 patients treated with 3D radiotherapy or IMRT after P/D. In the P/D-IMRT group, 65% of the 78 patients received > 45 Gy. The 2-year local failure rate was high at 60%, and there was one case each of grade 4 and grade 5 pneumonitis[123].
In a study of 24 patients treated with P/D and IMRT at a dose of 45 Gy with or without an integrated boost up to 57.5–60 Gy at the MDACC, there were no cases of grade 4 or 5 pulmonary toxicities, the median OS was 28 mo, and the crude local failure rate was 42%[98].
Shaaban et al[124] analyzed data from the National Cancer Database for 286 patients with MPM treated with IMRT. The median survival in patients treated with P/D and IMRT (63% of total) was 19 mo, but the difference with those treated with P/D and 3D radiotherapy was not significant. No information was given on toxicities.
Differences in the characteristics of surgery, chemotherapy, and radiotherapy may explain the heterogeneous results observed in the different series.
In conclusion, the combination of P/D and IMRT achieves acceptable disease control and is associated with a low rate of severe toxicities at radiation doses of 45–54 Gy[100]. IMRT can therefore be considered as an adjuvant therapy to P/D in well-selected patients. Enrolment for a phase III trial (NRG-LU006) comparing P/D plus chemotherapy with or without adjuvant IMRT (or intensity-modulated proton therapy) is expected to begin shortly.
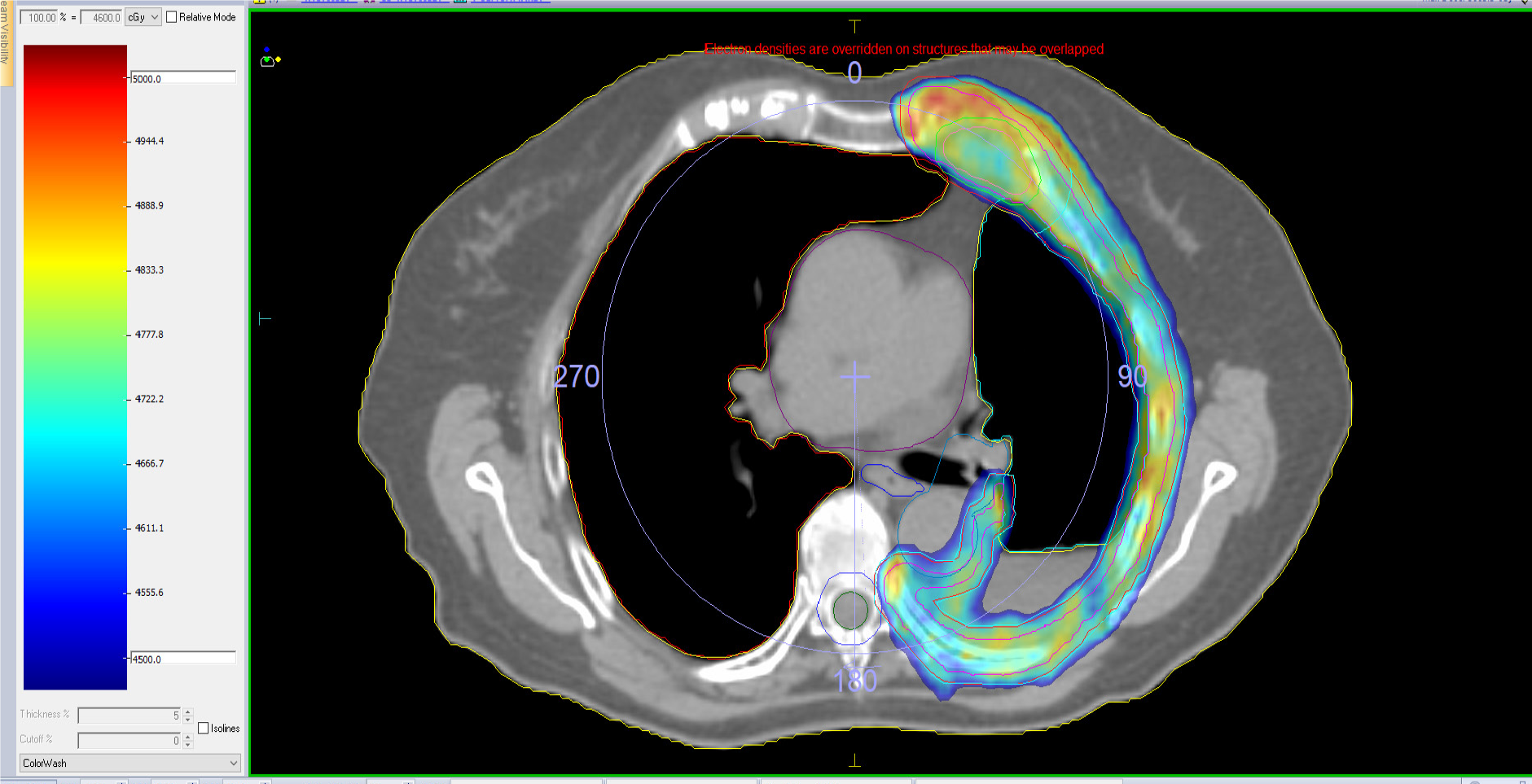
Refined IMRT techniques were followed by VMAT and HT, whose superior technical and dosimetric qualities may offer even better outcomes in the setting of MPM (Figures 3 and 4)
VMAT involves the delivery of 6–10-MV-photon beams in a rotating arc, thereby offering better dose conformality compared with IMRT in addition to shorter delivery times and increased safety. HT uses a linear accelerator incorporated into a CT unit. It is a dynamic, rotational technique (the table moves during treatment) that uses multiple “fan-like” beams conformed at 51 different angles using an automated multileaf collimator. The photons generated in the linear accelerator have an energy of 6 MV. Both VMAT and HT offer high precision, high conformality, and daily IGRT.
Comparative dosimetric studies have confirmed the theoretical advantage of VMAT and HT over IMRT[125-128].
Dumane et al[126] reported shorter treatment times for VMAT vs IMRT (8 vs 20 min) in addition to better sparing of OARs. Krayenbuehl et al[127], in turn, found that VMAT allowed for the use of lower lung doses and that two partial arcs yielded the best dosimetry.
Kimura et al[129] published the first study on the efficacy and safety of VMAT following EPP in MPM. They analyzed 15 patients treated with 54 Gy in 30 fractions and reported a 1-year local control rate of 55.7% and an OS rate of 43.1%. Patients with non-epithelioid MPM had worse local control rates. Three patients (20%) developed grade 3 pneumonitis.
Sterzing et al[128], in a dosimetric study comparing HT and step-and-shoot IMRT, found that the former offered better target coverage and homogeneity and also allowed for the delivery of a mean dose of less than 5 Gy to the contralateral lung. Thieke et al[110], by contrast, found no significant differences in dosimetry or clinical outcomes between HT and step-and-shoot IMRT.
Minatel et al[130] conducted a prospective study to evaluate the safety of HT in patients with MPM treated with P/D. In an initial analysis, they reported the results for 28 patients treated with 50 Gy in 25 fractions with a simultaneous integrated boost up to 60 Gy (2.4 Gy/fraction) delivered to fluorodeoxyglucose-avid areas observed by PET. There were five cases of grade 2 or 3 pneumonitis. The authors found that contralateral lung V5 was strongly correlated with the risk of pneumonitis and therefore restricted this parameter to 17% for the contralateral lung. In a subsequent analysis of 69 patients, they observed seven cases of grade 3 pneumonitis, one case of grade 5 pneumonitis, and good disease control (local failure rate, 19%; 2-year OS rate, 58%–65%).
Now that technological advances enable better dose distribution control, new studies have emerged to analyze the feasibility of escalating doses without increasing toxicity. Parisi et al[131] analyzed 36 patients treated with HT: 19 after P/D and 17 with unresectable disease who had undergone biopsy only. The median dose was 25 Gy (range 25–30 Gy) delivered in five fractions. The results were very promising. The median survival for a median follow-up of 37 mo was 22 mo, and there were three cases of grade 3 pneumonitis and no cases of grade 4 or 5 pneumonitis.
In a study by Fodor et al[132], 51 patients were treated with hypofractionated HT at a dose of 56 Gy and most of them received a concomitant boost up to 62.5 Gy. There were no grade 3 pulmonary toxicities in the group that did not receive a boost. In the group that did receive the boost, however, there were two cases of grade 5 toxicity and 11 cases of grade 3 toxicity. Median OS was 26 mo.
Pehlivan et al[133] compared VMAT and HT in patients with unresectable MPM. They concluded that both techniques offered efficient target volume coverage while maintaining OAR doses under established limits, although HT was associated with better mean and maximum doses and a significantly more homogeneous dose distribution (P < 0.001). The main drawback of HT was its longer treatment time (7.4 vs 2.5 min/fraction, P < 0.001).
In conclusion, although more studies and clinical trials are needed, both VMAT and HT can improve local control in MPM and also have an acceptable toxicity profile. VMAT and HT should be performed in experienced centers.
High local recurrence is a major problem in MPM and could perhaps be improved through a more radical strategy targeting pleural and regional recurrences.
SBRT involves the delivery of high-dose radiotherapy in just a few sessions. It is a high-precision, highly conformal technique that uses a steep dose gradient. The goal is to deliver a highly targeted ablative dose to the tumor.
A Swiss group of researchers retrospectively evaluated the feasibility of SBRT for pleural recurrences in patients with oligoprogressive MPM[134]. They analyzed the data for 50 lesions in 21 patients who developed local recurrence after initial treatment of MPM. The patients received a median of five fractions (range 3–20) with a median dose of 5 Gy/fraction (range 2.5–12.5 Gy). The total median dose was 30 Gy (range 20–50 Gy). Median follow-up from diagnosis was 28 mo (range 7–152 mo). The local control rate achieved (calculated by lesion) was 73.5%. Median DFS was 6 mo (range 0–21 mo), and median OS from the first SBRT session was 29 mo (range 0–61 mo). Just one patient experienced grade 3 or higher toxicities. SBRT was well tolerated, even after multiple repetitions, and it was associated with high local control rates and promising OS.
SRBT may be a promising option for achieving local control and delaying the need for systemic therapy in selected patients with a low tumor burden.
Radiotherapy can also be delivered using heavy particles with radiobiological properties, such as protons, carbon ions, neutrons, and alpha particles. These particles deposit a high dose to the tumor but doses to adjacent tissues rapidly fall off (Bragg peak). The main challenge with proton therapy is that proton beams are sensitive to volume and changing densities (air cavities) in the tissues they cross and are also altered by respiratory and cardiac movements.
Few studies have analyzed the usefulness of proton therapy in MPM.
Krayenbuehl et al[135] compared dosimetric planning for IMRT and proton therapy in patients with MPM treated with radical surgery (EPP). They found that proton therapy provided better PTV dose coverage and homogeneity in relation to V95 (P = 0.04) and also delivered a lower dose to OARs (kidneys, contralateral lung, heart, spinal cord, liver). Dose distributions, however, were very sensitive to changing air cavities.
Researchers at the University of Pennsylvania evaluated the use of proton therapy in 16 patients with MPM after radical pleurectomy[136]. Doses were calculated as cobalt gray equivalents (CGE). The median dose was 51.75 CGE (range 50.0–75.0 CGE) in daily fractions of 2.0 CGE (range 1.8–2.5 CGE). With a median follow-up of over 5 mo, all the patients achieved local control. There were no cases of acute or late grade 3 or higher toxicities.
The same group performed the first prospective study of proton therapy with adjuvant or salvage intent (eight and two patients, respectively)[137]. The median dose was 55.0 CGE/1.8–2.0 CGE (range 50–75 CGE). The 2-year local control rate was 90% and median survival was 19.5 mo from treatment completion and 30.3 mo from diagnosis. None of the patients experienced grade 2 or higher acute or late toxicity.
Evidence on the use of proton therapy in patients with MPM following P/D is even scarcer. Badiyan et al[138] studied 10 such patients treated with a mean total dose of 54 Gy (range 50.0-60.0/1.8-2.4 Gy). Median follow-up was 6.5 mo, and the 6-mo local control and OS rates were 87.5% and 64.3%, respectively. The treatment was well tolerated, and just two patients experienced grade 3 pneumonitis.
In conclusion, proton therapy following either EPP or P/D may offer dosimetric advantages[138]. Although no randomized controlled trials have yet been conducted, proton therapy can be contemplated for selected patients at centers with experience, preferably within the context of a clinical trial[139].
Adaptive radiotherapy involves the creation of new plans during the course of treatment to adapt to changes in target volumes detected by IGRT. These changes may be the result of anatomic variations (changes in tumor volume, patient weight/waist circumference), organ movement, or variations in patient positioning.
Adaptive radiotherapy is a potentially useful tool for tumors such as MPM where it is difficult to meet OAR dose constraints due to the large treatment volumes required. There is, however, very little experience with this modality in the setting of MPM. Considering the lack of evidence and the few descriptions of its use in clinical practice, adaptive radiotherapy should be used with caution in MPM until more robust evidence becomes available.
Patterns of recurrence in MPM vary widely between series. The most common pattern of recurrence is distant metastasis, which can occur alone or in association with local recurrence[107].
Distant metastasis was the most common pattern in the MDACC series of patients treated with EPP followed by hemithoracic IMRT. It occurred alone in 59% of cases and in association with local recurrence in 16%[109]. Just two patients (2.3%) developed local recurrence only. The most common site for locoregional recurrence was the ipsilateral hemithorax. Up to 57% of local recurrences were located in a previous radiotherapy field.
In the only prospective randomized controlled trial to analyze radiotherapy after EPP (SAKK 17/046), the recurrence rates were 5% for local recurrence only (within the PTV), 19% for synchronous distant and local recurrence, and 62% for distant metastasis only (outside the PTV)[92].
Based on data from small retrospective series with short follow-up times, recurrence patterns following P/D seem to differ somewhat from those seen in EPP, with higher rates of local recurrence (64% vs 31%) and lower rates of distant metastasis (36% vs 69%).
Very little has been published on the treatment of recurrence in MPM. Recurrence is a marker of poor prognosis (median survival of 3-mo following detection). In an analysis of 106 patients with recurrent disease, 74%were treated with salvage therapy (single modality in 86% of cases and a combination of treatments in 14%). The most common option was chemotherapy (71%) followed by radiotherapy (23%) and surgery (21%)[140]. The use of salvage therapy in these cases significantly increased DFS (10 vs 2 mo) (P < 0.0005).
The evidence on salvage surgery in MPM is controversial due to highly heterogeneous results and the use of small samples and different techniques. Salvage surgery can be contemplated on a case-by-case basis for patients who present exclusively with local recurrence amenable to surgery following trimodality therapy.
In a retrospective series of recurrence patterns and second-line treatments following multimodality therapy, 21% of patients presenting with local recurrence were treated with surgery, which was associated with an OS of 16 mo and significantly longer recurrence-free survival than either chemotherapy or radiotherapy (P < 0.0005)[140]. Resection of recurrent disease at the ipsilateral chest wall (9 patients) and extended chest-wall resections (4 patients) were safe and effective. Contralateral partial pleurectomy, by contrast (3 patients), was associated with high morbidity and mortality and is not recommended.
Reradiation can be considered for the treatment of asymptomatic local recurrence. Doses and fractionation should be planned according to the site and extension of the tumor and the characteristics of the patient[28] (type of recommendation: Informal consensus, moderate level of recommendation).
Salvage radiotherapy has been administered using conventional (standard fractionation) or hypofractionated protocols, as monotherapy, and in combination with chemotherapy or after salvage surgery (positive margins). Heterogeneous outcomes have been reported.
The treatment of oligoprogressive disease is challenging, and an optimal approach has yet to be defined. Ablative treatment, however, may increase local recurrence–free survival. Based on results from other settings, SBRT may be a promising option for salvage radiotherapy in patients with oligoprogressive disease[134].
Salvage chemotherapy provides poor results (median survival of 3–7 mo after recurrence)[29,141,142]. It can be considered in patients in good physical condition (ECOG ≤ 2) with unresectable local disease or distant metastasis. Patients who achieve a lasting response (> 6 mo) to induction chemotherapy with cisplatin/pemetrexed can be given the option of repeating this treatment or switching to vinorelbine, which is associated with better tolerance. Enrollment in a clinical trial should be considered given the limited efficacy of salvage chemotherapy.
Promising preliminary results for immunotherapy (pembrolizumab monotherapy or nivolumab +/- ipilimumab) have been observed in MPM since the first studies were published in 2009[29]. Two phase III trials (NCT02991482 and NCT03063450) are currently underway, but no results have been reported yet.
There are no clear evidence-based recommendations on post-treatment follow-up of patients with MPM. Many guidelines, including the European Society of Medical Oncology guidelines[30], recommend an individualized approach, determined by the patient's medical team. Signs or symptoms detected during follow-up, such as reduced respiratory function, chest pain, cough, anorexia, weight loss, and asthenia, can raise suspicion of disease progression. Nonetheless, some clinical guidelines recommend a CT scan every 3 to 6 mo after completion of active treatment[143]. The role of PET or MRI during the follow-up of patients with MPM is unclear. The development of targeted therapies and immunotherapy, which normally occurs within a clinical trial setting, will probably give rise to more objective follow-up strategies in the future[144].
A 62-year-old male patient with a personal history of heavy ex-smoking of 20-30 cigarettes per day for 30 years until 2005. Occupational exposure to asbestos for 25 years. Melanoma in left lower limb treated with surgery.
Consultation in February 2020 for discrete increase of dyspnea without other significant associated symptoms. CT and PET-CT showed mild left pleural effusion and left pleural thickening predominantly in the apical area with suspected mesothelioma as the first option. The biopsy was positive for malignant mesothelioma.
The patient presented good respiratory functional data, so as it was a localized disease; treatment with neoadjuvant chemotherapy and subsequent surgery was considered by the tumor committee.
The patient presented a partial response to chemotherapy and non-radical lung-sparing surgery was proposed, obtaining a complete resection.
The pathological anatomy showed an epithelioid malignant mesothelioma, with disease predominating in the left latero-apical area, and two foci of disease in the caudal and medial area of the pleura.
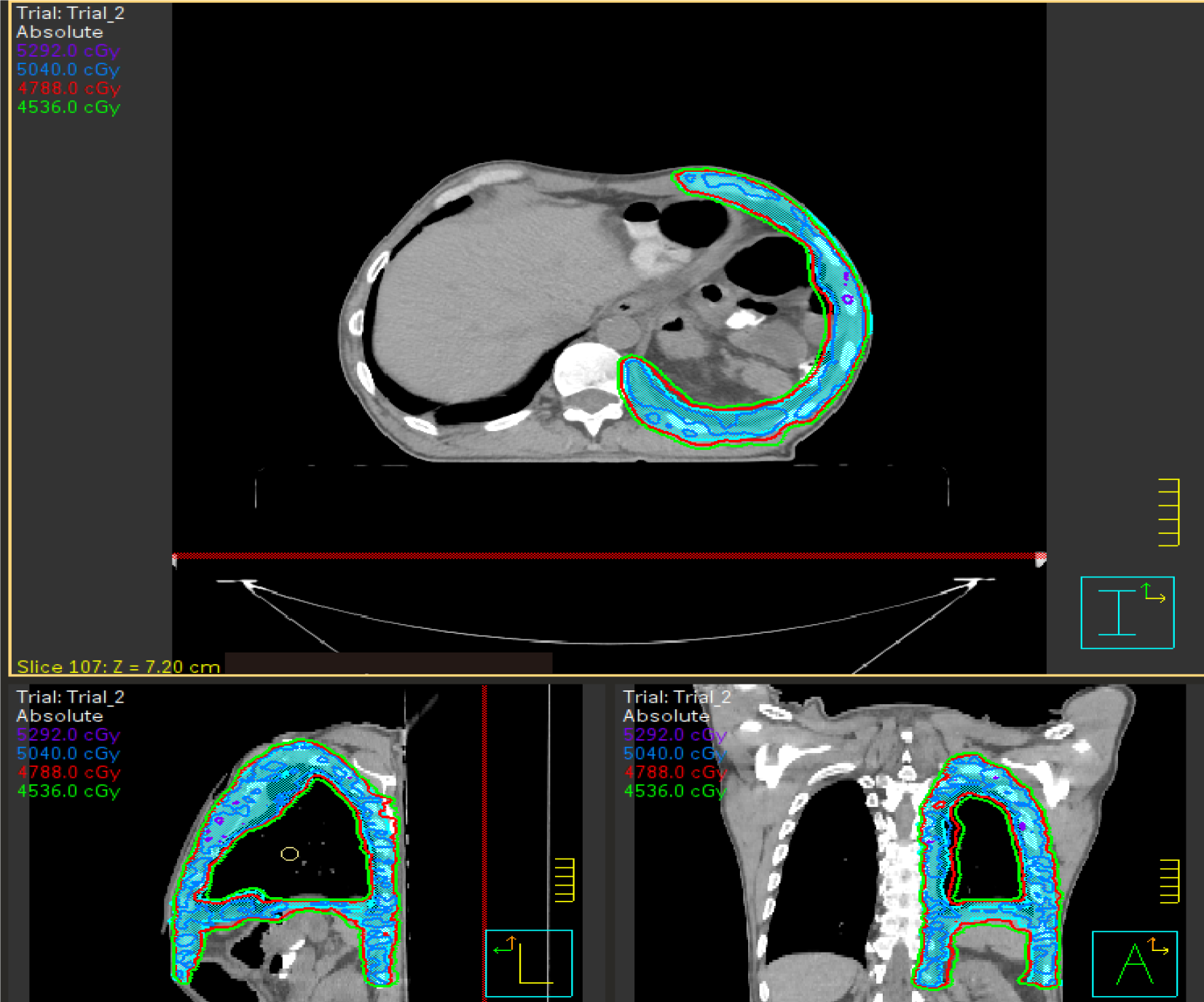
Treatment was proposed with adjuvant RTE, with VMAT technique, with a dose of 46 Gy on total left hemipleura and a boost in areas of tumor persistence after chemotherapy up to 60 Gy, with integrated boost technique, treatment in 23 sessions (Figures 5 and 6)
The established dosimetric criteria were met (Table 5), and the patient presented good tolerance to treatment, with the appearance of pleural effusion without associated symptoms during treatment.
MPM is a rare tumor that is very challenging to treat. Advances in surgery and radiotherapy, together with the development of new systemic therapies, have brought modest improvements in survival and local control and considerably lower toxicity than classic treatments Adequately powered, homogeneous clinical studies evaluating these advances are needed to provide objective evidence of improvements and clear evidence-based guidelines for the management of this disease.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Doi H S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, Gnad F, Nguyen TT, Jaiswal BS, Chirieac LR, Sciaranghella D, Dao N, Gustafson CE, Munir KJ, Hackney JA, Chaudhuri A, Gupta R, Guillory J, Toy K, Ha C, Chen YJ, Stinson J, Chaudhuri S, Zhang N, Wu TD, Sugarbaker DJ, de Sauvage FJ, Richards WG, Seshagiri S. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 600] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 2. | Yap TA, Aerts JG, Popat S, Fennell DA. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer. 2017;17:475-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 3. | Scherpereel A, Wallyn F, Albelda SM, Munck C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018;19:e161-e172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 4. | Kameda T, Takahashi K, Kim R, Jiang Y, Movahed M, Park EK, Rantanen J. Asbestos: use, bans and disease burden in Europe. Bull World Health Organ. 2014;92:790-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Lin RT, Takahashi K, Karjalainen A, Hoshuyama T, Wilson D, Kameda T, Chan CC, Wen CP, Furuya S, Higashi T, Chien LC, Ohtaki M. Ecological association between asbestos-related diseases and historical asbestos consumption: an international analysis. Lancet. 2007;369:844-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer. 1999;79:666-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 579] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 7. | Trama A, Marcos-Gragera R, Sánchez Pérez MJ, van der Zwan JM, Ardanaz E, Bouchardy C, Melchor JM, Martinez C, Capocaccia R, Vicentini M, Siesling S, Gatta G; RARECARE working group contributing to the data quality study. Data quality in rare cancers registration: the report of the RARECARE data quality study. Tumori. 2017;103:22-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Berman DW, Crump KS. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit Rev Toxicol. 2008;38 Suppl 1:49-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Aguilar-Madrid G, Robles-Pérez E, Juárez-Pérez CA, Alvarado-Cabrero I, Rico-Méndez FG, Javier KG. Case-control study of pleural mesothelioma in workers with social security in Mexico. Am J Ind Med. 2010;53:241-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Rushton L, Bagga S, Bevan R, Brown TP, Cherrie JW, Holmes P, Fortunato L, Slack R, Van Tongeren M, Young C, Hutchings SJ. Occupation and cancer in Britain. Br J Cancer. 2010;102:1428-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Lacourt A, Gramond C, Rolland P, Ducamp S, Audignon S, Astoul P, Chamming's S, Gilg Soit Ilg A, Rinaldo M, Raherison C, Galateau-Salle F, Imbernon E, Pairon JC, Goldberg M, Brochard P. Occupational and non-occupational attributable risk of asbestos exposure for malignant pleural mesothelioma. Thorax. 2014;69:532-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Ferrante D, Mirabelli D, Tunesi S, Terracini B, Magnani C. Pleural mesothelioma and occupational and non-occupational asbestos exposure: a case-control study with quantitative risk assessment. Occup Environ Med. 2016;73:147-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Metintas M, Hillerdal G, Metintas S, Dumortier P. Endemic malignant mesothelioma: exposure to erionite is more important than genetic factors. Arch Environ Occup Health. 2010;65:86-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Ortega-Guerrero MA, Carrasco-Núñez G. Environmental occurrence, origin, physical and geochemical properties, and carcinogenic potential of erionite near San Miguel de Allende, Mexico. Environ Geochem Health. 2014;36:517-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Bruno C, Tumino R, Fazzo L, Cascone G, Cernigliaro A, De Santis M, Giurdanella MC, Nicita C, Rollo PC, Scondotto S, Spata E, Zona A, Comba P. Incidence of pleural mesothelioma in a community exposed to fibres with fluoro-edenitic composition in Biancavilla (Sicily, Italy). Ann Ist Super Sanita. 2014;50:111-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 16. | Ortega-Guerrero MA, Carrasco-Núñez G, Barragán-Campos H, Ortega MR. High incidence of lung cancer and malignant mesothelioma linked to erionite fibre exposure in a rural community in Central Mexico. Occup Environ Med. 2015;72:216-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Van Gosen BS, Blitz TA, Plumlee GS, Meeker GP, Pierson MP. Geologic occurrences of erionite in the United States: an emerging national public health concern for respiratory disease. Environ Geochem Health. 2013;35:419-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Carbone M, Baris YI, Bertino P, Brass B, Comertpay S, Dogan AU, Gaudino G, Jube S, Kanodia S, Partridge CR, Pass HI, Rivera ZS, Steele I, Tuncer M, Way S, Yang H, Miller A. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc Natl Acad Sci USA. 2011;108:13618-13623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Ji J, Sundquist J, Sundquist K. Incidence and familial risk of pleural mesothelioma in Sweden: a national cohort study. Eur Respir J. 2016;48:873-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Ascoli V, Romeo E, Carnovale Scalzo C, Cozzi I, Ancona L, Cavariani F, Balestri A, Gasperini L, Forastiere F. Familial malignant mesothelioma: a population-based study in central Italy (1980-2012). Cancer Epidemiol. 2014;38:273-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | de Klerk N, Alfonso H, Olsen N, Reid A, Sleith J, Palmer L, Berry G, Musk AB. Familial aggregation of malignant mesothelioma in former workers and residents of Wittenoom, Western Australia. Int J Cancer. 2013;132:1423-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, Hesdorffer M, Nasu M, Powers A, Rivera Z, Comertpay S, Tanji M, Gaudino G, Yang H, Carbone M. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 727] [Cited by in F6Publishing: 717] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 23. | Cheung M, Talarchek J, Schindeler K, Saraiva E, Penney LS, Ludman M, Testa JR. Further evidence for germline BAP1 mutations predisposing to melanoma and malignant mesothelioma. Cancer Genet. 2013;206:206-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Panou V, Gadiraju M, Wolin A, Weipert CM, Skarda E, Husain AN, Patel JD, Rose B, Zhang SR, Weatherly M, Nelakuditi V, Knight Johnson A, Helgeson M, Fischer D, Desai A, Sulai N, Ritterhouse L, Røe OD, Turaga KK, Huo D, Segal J, Kadri S, Li Z, Kindler HL, Churpek JE. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J Clin Oncol. 2018;36:2863-2871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 137] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 25. | Hassan R, Morrow B, Thomas A, Walsh T, Lee MK, Gulsuner S, Gadiraju M, Panou V, Gao S, Mian I, Khan J, Raffeld M, Patel S, Xi L, Wei JS, Hesdorffer M, Zhang J, Calzone K, Desai A, Padiernos E, Alewine C, Schrump DS, Steinberg SM, Kindler HL, King MC, Churpek JE. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci USA. 2019;116:9008-9013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 26. | Woolhouse I, Bishop L, Darlison L, De Fonseka D, Edey A, Edwards J, Faivre-Finn C, Fennell DA, Holmes S, Kerr KM, Nakas A, Peel T, Rahman NM, Slade M, Steele J, Tsim S, Maskell NA. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax. 2018;73:i1-i30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 27. | National Comprehensive Cancer Network (NCCN). Malignant Pleural Mesothelioma Guidelines Version 2020. [cited 20 April, 2021]. Available from: www.nccn.org/professionals/physician_gls/pdf/mpm_blocks.pdf. [Cited in This Article: ] |
| 28. | Kindler HL, Ismaila N, Armato SG 3rd, Bueno R, Hesdorffer M, Jahan T, Jones CM, Miettinen M, Pass H, Rimner A, Rusch V, Sterman D, Thomas A, Hassan R. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1343-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 252] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 29. | Scherpereel A, Opitz I, Berghmans T, Psallidas I, Glatzer M, Rigau D, Astoul P, Bölükbas S, Boyd J, Coolen J, De Bondt C, De Ruysscher D, Durieux V, Faivre-Finn C, Fennell D, Galateau-Salle F, Greillier L, Hoda MA, Klepetko W, Lacourt A, McElnay P, Maskell NA, Mutti L, Pairon JC, Van Schil P, van Meerbeeck JP, Waller D, Weder W, Cardillo G, Putora PM. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J. 2020;55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 30. | Baas P, Fennell D, Kerr KM, Van Schil PE, Haas RL, Peters S; ESMO Guidelines Committee. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v31-v39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 31. | Khan AR, Khan S, Zimmerman V, Baddour LM, Tleyjeh IM. Quality and strength of evidence of the Infectious Diseases Society of America clinical practice guidelines. Clin Infect Dis. 2010;51:1147-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P, Dienemann H, Galateau-Salle F, Hennequin C, Hillerdal G, Le Péchoux C, Mutti L, Pairon JC, Stahel R, van Houtte P, van Meerbeeck J, Waller D, Weder W; European Respiratory Society/European Society of Thoracic Surgeons Task Force. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 33. | Zahid I, Sharif S, Routledge T, Scarci M. What is the best way to diagnose and stage malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Erasmus JJ, Truong MT, Smythe WR, Munden RF, Marom EM, Rice DC, Vaporciyan AA, Walsh GL, Sabloff BS, Broemeling LD, Stevens CW, Pisters KM, Podoloff DA, Macapinlac HA. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: Staging implications. J Thorac Cardiovasc Surg. 2005;129:1364-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Poe RH, Israel RH, Utell MJ, Hall WJ, Greenblatt DW, Kallay MC. Sensitivity, specificity, and predictive values of closed pleural biopsy. Arch Intern Med. 1984;144:325-328. [PubMed] [Cited in This Article: ] |
| 36. | Von Hoff DD, LiVolsi V. Diagnostic reliability of needle biopsy of the parietal pleura. A review of 272 biopsies. Am J Clin Pathol. 1975;64:200-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Hooper C, Lee YC, Maskell N; BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii4-i17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 402] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 38. | Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology. 2001;219:510-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomized controlled trial. Lancet. 2003;361:1326-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 40. | Heilo A, Stenwig AE, Solheim OP. Malignant pleural mesothelioma: US-guided histologic core-needle biopsy. Radiology. 1999;211:657-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Rahman NM, Ali NJ, Brown G, Chapman SJ, Davies RJ, Downer NJ, Gleeson FV, Howes TQ, Treasure T, Singh S, Phillips GD; British Thoracic Society Pleural Disease Guideline Group. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii54-ii60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 42. | Haridas N, K P S, T P R, P T J, Chetambath R. Medical Thoracoscopy vs Closed Pleural Biopsy in Pleural Effusions: A Randomized Controlled Study. J Clin Diagn Res. 2014;8:MC01-MC04. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Maturu VN, Dhooria S, Bal A, Singh N, Aggarwal AN, Gupta D, Behera D, Agarwal R. Role of medical thoracoscopy and closed-blind pleural biopsy in undiagnosed exudative pleural effusions: a single-center experience of 348 patients. J Bronchology Interv Pulmonol. 2015;22:121-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Tscheikuna J. Medical thoracoscopy: experiences in Siriraj Hospital. J Med Assoc Thai. 2006;89 Suppl 5:S62-S66. [PubMed] [Cited in This Article: ] |
| 45. | de Groot M, Walther G. Thoracoscopy in undiagnosed pleural effusions. S Afr Med J. 1998;88:706-711. [PubMed] [Cited in This Article: ] |
| 46. | Meyerhoff RR, Yang CF, Speicher PJ, Gulack BC, Hartwig MG, D'Amico TA, Harpole DH, Berry MF. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res. 2015;196:23-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 47. | Kadota K, Suzuki K, Sima CS, Rusch VW, Adusumilli PS, Travis WD. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol. 2011;6:896-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, Butnor KJ, Chirieac LR, Churg AM, Dacic S, Galateau-Sallé F, Gibbs A, Gown AM, Krausz T, Litzky LA, Marchevsky A, Nicholson AG, Roggli VL, Sharma AK, Travis WD, Walts AE, Wick MR. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018;142:89-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 362] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 49. | Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, Borczuk AC, Butnor K, Cagle PT, Chirieac LR, Churg A, Dacic S, Fraire A, Galateau-Salle F, Gibbs A, Gown A, Hammar S, Litzky L, Marchevsky AM, Nicholson AG, Roggli V, Travis WD, Wick M; International Mesothelioma Interest Group. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137:647-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 50. | Ordóñez NG. The diagnostic utility of immunohistochemistry in distinguishing between epithelioid mesotheliomas and squamous carcinomas of the lung: a comparative study. Mod Pathol. 2006;19:417-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Galateau-Sallé F, Vignaud JM, Burke L, Gibbs A, Brambilla E, Attanoos R, Goldberg M, Launoy G; Mesopath group. Well-differentiated papillary mesothelioma of the pleura: a series of 24 cases. Am J Surg Pathol. 2004;28:534-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Nowak AK, Chansky K, Rice DC, Pass HI, Kindler HL, Shemanski L, Billé A, Rintoul RC, Batirel HF, Thomas CF, Friedberg J, Cedres S, de Perrot M, Rusch VW; Staging and Prognostic Factors Committee; Advisory Boards and Participating Institutions. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol. 2016;11:2089-2099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 53. | Rusch VW, Chansky K, Kindler HL, Nowak AK, Pass HI, Rice DC, Shemanski L, Galateau-Sallé F, McCaughan BC, Nakano T, Ruffini E, van Meerbeeck JP, Yoshimura M; IASLC Staging and Prognostic Factors Committee; advisory boards, and participating institutions. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol. 2016;11:2112-2119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 54. | Rice D, Chansky K, Nowak A, Pass H, Kindler H, Shemanski L, Opitz I, Call S, Hasegawa S, Kernstine K, Atinkaya C, Rea F, Nafteux P, Rusch VW; Mesothelioma Domain of the IASLC Staging and Prognostic Factors Committee; advisory boards and participating institutions. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol. 2016;11:2100-2111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 55. | Baldini EH. Radiation therapy options for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009;21:159-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Rice DC, Smythe WR, Liao Z, Guerrero T, Chang JY, McAleer MF, Jeter MD, Correa A, Vaporciyan AA, Liu HH, Komaki R, Forster KM, Stevens CW. Dose-dependent pulmonary toxicity after postoperative intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2007;69:350-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Rintoul RC, Ritchie AJ, Edwards JG, Waller DA, Coonar AS, Bennett M, Lovato E, Hughes V, Fox-Rushby JA, Sharples LD; MesoVATS Collaborators. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomized, controlled trial. Lancet. 2014;384:1118-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 58. | Rice D, Rusch V, Pass H, Asamura H, Nakano T, Edwards J, Giroux DJ, Hasegawa S, Kernstine KH, Waller D, Rami-Porta R; International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol. 2011;6:1304-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 59. | Nelson DB, Rice DC, Mitchell KG, Tsao AS, Gomez DR, Sepesi B, Mehran RJ. Return to intended oncologic treatment after surgery for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2019;158:924-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Flores RM, Pass HI, Seshan VE, Dycoco J, Zakowski M, Carbone M, Bains MS, Rusch VW. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 61. | de Perrot M, Feld R, Cho BC, Bezjak A, Anraku M, Burkes R, Roberts H, Tsao MS, Leighl N, Keshavjee S, Johnston MR. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:1413-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 62. | Weder W, Stahel RA, Bernhard J, Bodis S, Vogt P, Ballabeni P, Lardinois D, Betticher D, Schmid R, Stupp R, Ris HB, Jermann M, Mingrone W, Roth AD, Spiliopoulos A; Swiss Group for Clinical Cancer Research. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol. 2007;18:1196-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 63. | Van Schil PE, Baas P, Gaafar R, Maat AP, Van de Pol M, Hasan B, Klomp HM, Abdelrahman AM, Welch J, van Meerbeeck JP; European Organisation for Research and Treatment of Cancer (EORTC) Lung Cancer Group. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J. 2010;36:1362-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 64. | Krug LM, Pass HI, Rusch VW, Kindler HL, Sugarbaker DJ, Rosenzweig KE, Flores R, Friedberg JS, Pisters K, Monberg M, Obasaju CK, Vogelzang NJ. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:3007-3013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 65. | Cao CQ, Yan TD, Bannon PG, McCaughan BC. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol. 2010;5:1692-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 66. | Treasure T, Lang-Lazdunski L, Waller D, Bliss JM, Tan C, Entwisle J, Snee M, O'Brien M, Thomas G, Senan S, O'Byrne K, Kilburn LS, Spicer J, Landau D, Edwards J, Coombes G, Darlison L, Peto J; MARS trialists. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 474] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 67. | Cao C, Tian D, Manganas C, Matthews P, Yan TD. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann Cardiothorac Surg. 2012;1:428-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 33] [Reference Citation Analysis (0)] |
| 68. | Trovo M, Relevant A, Polesel J, Muraro E, Barresi L, Drigo A, Baresic T, Bearz A, Fanetti G, Del Conte A, Matrone F, Reverberi C, Furlan C, Zuccon U, Fontana P, Franchin G, Minatel E. Radical Hemithoracic Radiotherapy Versus Palliative Radiotherapy in Non-metastatic Malignant Pleural Mesothelioma: Results from a Phase 3 Randomized Clinical Trial. Int J Radiat Oncol Biol Phys. 2021;109:1368-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Cho BC, Feld R, Leighl N, Opitz I, Anraku M, Tsao MS, Hwang DM, Hope A, de Perrot M. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: the "SMART" approach for resectable malignant pleural mesothelioma. J Thorac Oncol. 2014;9:397-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 70. | de Perrot M, Feld R, Leighl NB, Hope A, Waddell TK, Keshavjee S, Cho BC. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2016;151:468-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 71. | Stahel RA, Riesterer O, Xyrafas A, Opitz I, Beyeler M, Ochsenbein A, Früh M, Cathomas R, Nackaerts K, Peters S, Mamot C, Zippelius A, Mordasini C, Caspar CB, Eckhardt K, Schmid RA, Aebersold DM, Gautschi O, Nagel W, Töpfer M, Krayenbuehl J, Ribi K, Ciernik IF, Weder W. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol. 2015;16:1651-1658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 72. | Nelson DB, Rice DC, Mitchell KG, Tsao AS, Vaporciyan AA, Antonoff MB, Hofstetter WL, Walsh GL, Swisher SG, Roth JA, Gomez DR, Mehran RJ, Sepesi B. Defining the role of adjuvant radiotherapy for malignant pleural mesothelioma: a propensity-matched landmark analysis of the National Cancer Database. J Thorac Dis. 2019;11:1269-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | O'Rourke N, Garcia JC, Paul J, Lawless C, McMenemin R, Hill J. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol. 2007;84:18-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Bayman N, Appel W, Ashcroft L, Baldwin DR, Bates A, Darlison L, Edwards JG, Ezhil V, Gilligan D, Hatton M, Jegannathen A, Mansy T, Peake MD, Pemberton L, Rintoul RC, Snee M, Ryder WD, Taylor P, Faivre-Finn C. Prophylactic Irradiation of Tracts in Patients With Malignant Pleural Mesothelioma: An Open-Label, Multicenter, Phase III Randomized Trial. J Clin Oncol. 2019;37:1200-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 75. | Clive AO, Taylor H, Dobson L, Wilson P, de Winton E, Panakis N, Pepperell J, Howell T, Stewart SA, Penz E, Jordan N, Morley AJ, Zahan-Evans N, Smith S, Batchelor TJP, Marchbank A, Bishop L, Ionescu AA, Bayne M, Cooper S, Kerry A, Jenkins P, Toy E, Vigneswaran V, Gildersleve J, Ahmed M, McDonald F, Button M, Lewanski C, Comins C, Dakshinamoorthy M, Lee YCG, Rahman NM, Maskell NA. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): a multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol. 2016;17:1094-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 76. | Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest. 1995;108:754-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 371] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 77. | Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636-2644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2274] [Cited by in F6Publishing: 2181] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 78. | Zellos L, Christiani DC. Epidemiology, biologic behavior, and natural history of mesothelioma. Thorac Surg Clin. 2004;14:469-477, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Macleod N, Price A, O'Rourke N, Fallon M, Laird B. Radiotherapy for the treatment of pain in malignant pleural mesothelioma: a systematic review. Lung Cancer. 2014;83:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | MacLeod N, Chalmers A, O'Rourke N, Moore K, Sheridan J, McMahon L, Bray C, Stobo J, Price A, Fallon M, Laird BJ. Is Radiotherapy Useful for Treating Pain in Mesothelioma? J Thorac Oncol. 2015;10:944-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 81. | Ashton M, O'Rourke N, Macleod N, Laird B, Stobo J, Kelly C, Alexander L, Franks K, Moore K, Currie S, Valentine R, Chalmers AJ. SYSTEMS-2: A randomised phase II study of radiotherapy dose escalation for pain control in malignant pleural mesothelioma. Clin Transl Radiat Oncol. 2018;8:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, Legrand C, Bottomley A, Debruyne C, Giaccone G; European Organisation for Research and Treatment of Cancer Lung Cancer Group; National Cancer Institute of Canada. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881-6889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 452] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 83. | Santoro A, O'Brien ME, Stahel RA, Nackaerts K, Baas P, Karthaus M, Eberhardt W, Paz-Ares L, Sundstrom S, Liu Y, Ripoche V, Blatter J, Visseren-Grul CM, Manegold C. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaïve patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol. 2008;3:756-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 84. | Ceresoli GL, Castagneto B, Zucali PA, Favaretto A, Mencoboni M, Grossi F, Cortinovis D, Del Conte G, Ceribelli A, Bearz A, Salamina S, De Vincenzo F, Cappuzzo F, Marangolo M, Torri V, Santoro A. Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: combined analysis of two phase II trials. Br J Cancer. 2008;99:51-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, Gounant V, Rivière F, Janicot H, Gervais R, Locher C, Milleron B, Tran Q, Lebitasy MP, Morin F, Creveuil C, Parienti JJ, Scherpereel A; French Cooperative Thoracic Intergroup (IFCT). Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 602] [Cited by in F6Publishing: 624] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 86. | Stebbing J, Powles T, McPherson K, Shamash J, Wells P, Sheaff MT, Slater S, Rudd RM, Fennell D, Steele JP. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer. 2009;63:94-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 87. | Zauderer MG, Kass SL, Woo K, Sima CS, Ginsberg MS, Krug LM. Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer. 2014;84:271-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 88. | Muers MF, Stephens RJ, Fisher P, Darlison L, Higgs CM, Lowry E, Nicholson AG, O'Brien M, Peake M, Rudd R, Snee M, Steele J, Girling DJ, Nankivell M, Pugh C, Parmar MK; MS01 Trial Management Group. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet. 2008;371:1685-1694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 89. | Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L, Maio M. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 261] [Article Influence: 23.7] [Reference Citation Analysis (3)] |
| 90. | Botticella A, Defraene G, Nackaerts K, Deroose CM, Coolen J, Nafteux P, Peeters S, Ricardi U, De Ruysscher D. Optimal gross tumor volume definition in lung-sparing intensity modulated radiotherapy for pleural mesothelioma: an in silico study. Acta Oncol. 2016;55:1450-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg. 2014;260:577-580; discussion 580-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 92. | Riesterer O, Ciernik IF, Stahel RA, Xyrafas A, Aebersold DM, Plasswilm L, Mahmut Ozsahin E, Zwahlen DR, Nackaerts K, Zimmermann F, Sabrina Stark L, Weder W, Krayenbuehl J; Swiss Group for Clinical Cancer Research (SAKK). Pattern of failure after adjuvant radiotherapy following extrapleural pneumonectomy of pleural mesothelioma in the SAKK 17/04 trial. Radiother Oncol. 2019;138:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Rea F, Marulli G, Bortolotti L, Breda C, Favaretto AG, Loreggian L, Sartori F. Induction chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic radiation in malignant pleural mesothelioma (MPM): Feasibility and results. Lung Cancer. 2007;57:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Ahamad A, Stevens CW, Smythe WR, Vaporciyan AA, Komaki R, Kelly JF, Liao Z, Starkschall G, Forster KM. Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2003;55:768-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 95. | Bece A, Tin MM, Martin D, Lin R, McLean J, McCaughan B. Hemithoracic radiation therapy after extrapleural pneumonectomy for malignant pleural mesothelioma: Toxicity and outcomes at an Australian institution. J Med Imaging Radiat Oncol. 2015;59:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 96. | Ebara T, Kawamura H, Kaminuma T, Okamoto M, Yoshida D, Okubo Y, Takahashi T, Kobayashi K, Sakaguchi H, Ando Y, Nakano T. Hemithoracic intensity-modulated radiotherapy using helical tomotherapy for patients after extrapleural pneumonectomy for malignant pleural mesothelioma. J Radiat Res. 2012;53:288-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Hasegawa S, Okada M, Tanaka F, Yamanaka T, Soejima T, Kamikonya N, Tsujimura T, Fukuoka K, Yokoi K, Nakano T. Trimodality strategy for treating malignant pleural mesothelioma: results of a feasibility study of induction pemetrexed plus cisplatin followed by extrapleural pneumonectomy and postoperative hemithoracic radiation (Japan Mesothelioma Interest Group 0601 Trial). Int J Clin Oncol. 2016;21:523-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 98. | Chance WW, Rice DC, Allen PK, Tsao AS, Fontanilla HP, Liao Z, Chang JY, Tang C, Pan HY, Welsh JW, Mehran RJ, Gomez DR. Hemithoracic intensity modulated radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma: toxicity, patterns of failure, and a matched survival analysis. Int J Radiat Oncol Biol Phys. 2015;91:149-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 99. | Yajnik S, Rosenzweig KE, Mychalczak B, Krug L, Flores R, Hong L, Rusch VW. Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2003;56:1319-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 100. | Rimner A, Zauderer MG, Gomez DR, Adusumilli PS, Parhar PK, Wu AJ, Woo KM, Shen R, Ginsberg MS, Yorke ED, Rice DC, Tsao AS, Rosenzweig KE, Rusch VW, Krug LM. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol. 2016;34:2761-2768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 101. | NRG Oncology. Center for Innovation in Radiation Oncology. [cited 20 April 2021]. Available from: https://www.nrgoncology.org/ciro-lung. [Cited in This Article: ] |
| 102. | Stevens CW, Forster KM, Smythe WR, Rice D. Radiotherapy for mesothelioma. Hematol Oncol Clin North Am. 2005;19:1099-1115, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | MacRae RM, Ashton M, Lauk O, Wilson W, O'Rourke N, Simone CB 2nd, Rimner A. The role of radiation treatment in pleural mesothelioma: Highlights of the 14th International Conference of the International mesothelioma interest group. Lung Cancer. 2019;132:24-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Gupta T, Agarwal J, Jain S, Phurailatpam R, Kannan S, Ghosh-Laskar S, Murthy V, Budrukkar A, Dinshaw K, Prabhash K, Chaturvedi P, D'Cruz A. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol. 2012;104:343-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 105. | Ulger S, Cetin E, Catli S, Sarac H, Kilic D, Bora H. Intensity-Modulated Radiation Therapy Improves the Target Coverage Over 3-D Planning While Meeting Lung Tolerance Doses for All Patients With Malignant Pleural Mesothelioma. Technol Cancer Res Treat. 2017;16:332-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 106. | Krayenbuehl J, Oertel S, Davis JB, Ciernik IF. Combined photon and electron three-dimensional conformal versus intensity-modulated radiotherapy with integrated boost for adjuvant treatment of malignant pleural mesothelioma after pleuropneumonectomy. Int J Radiat Oncol Biol Phys. 2007;69:1593-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | de Perrot M, Wu L, Wu M, Cho BCJ. Radiotherapy for the treatment of malignant pleural mesothelioma. Lancet Oncol. 2017;18:e532-e542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 108. | Bille A, Belcher E, Raubenheimer H, Landau D, Cane P, Spicer J, Lang-Lazdunski L. Induction chemotherapy, extrapleural pneumonectomy, and adjuvant radiotherapy for malignant pleural mesothelioma: experience of Guy's and St Thomas' hospitals. Gen Thorac Cardiovasc Surg. 2012;60:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 109. | Gomez DR, Hong DS, Allen PK, Welsh JS, Mehran RJ, Tsao AS, Liao Z, Bilton SD, Komaki R, Rice DC. Patterns of failure, toxicity, and survival after extrapleural pneumonectomy and hemithoracic intensity-modulated radiation therapy for malignant pleural mesothelioma. J Thorac Oncol. 2013;8:238-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 110. | Thieke C, Nicolay NH, Sterzing F, Hoffmann H, Roeder F, Safi S, Debus J, Huber PE. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol. 2015;10:267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 111. | Rice DC, Stevens CW, Correa AM, Vaporciyan AA, Tsao A, Forster KM, Walsh GL, Swisher SG, Hofstetter WL, Mehran RJ, Roth JA, Liao Z, Smythe WR. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2007;84:1685-1692; discussion 1692-1693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 112. | Buduhan G, Menon S, Aye R, Louie B, Mehta V, Vallières E. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2009;88:870-875; discussion 876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Rosenzweig KE. Malignant pleural mesothelioma: adjuvant therapy with radiation therapy. Ann Transl Med. 2017;5:242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 114. | Chi A, Liao Z, Nguyen NP, Howe C, Gomez D, Jang SY, Komaki R. Intensity-modulated radiotherapy after extrapleural pneumonectomy in the combined-modality treatment of malignant pleural mesothelioma. J Thorac Oncol. 2011;6:1132-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Allen AM, Czerminska M, Jänne PA, Sugarbaker DJ, Bueno R, Harris JR, Court L, Baldini EH. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 116. | Gomez DR, Rimner A, Simone CB 2nd, Cho BCJ, de Perrot M, Adjei AA, Bueno R, Gill RR, Harpole DH Jr, Hesdorffer M, Hirsch FR, Jackson AA, Pass HI, Rice DC, Rusch VW, Tsao AS, Yorke E, Rosenzweig K. The Use of Radiation Therapy for the Treatment of Malignant Pleural Mesothelioma: Expert Opinion from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol. 2019;14:1172-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 117. | Patel PR, Yoo S, Broadwater G, Marks LB, Miles EF, D'Amico TA, Harpole D, Kelsey CR. Effect of increasing experience on dosimetric and clinical outcomes in the management of malignant pleural mesothelioma with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83:362-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Gelzinis TA. The 2019 ERS/ESTS/EACTS/ESTRO Guidelines on the Management of Patients With Malignant Pleural Mesothelioma. J Cardiothorac Vasc Anesth. 2021;35:378-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Gupta V, Mychalczak B, Krug L, Flores R, Bains M, Rusch VW, Rosenzweig KE. Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2005;63:1045-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 120. | Patel R, Ludmir EB, Miccio JA, Menon H, Barsky AR, Mesko SM, Kodali M, Lautenschlaeger T, Adeberg S, Simone CB 2nd, Verma V. Disease-Related Outcomes and Toxicities of Intensity Modulated Radiation Therapy After Lung-Sparing Pleurectomy for Malignant Pleural Mesothelioma: A Systematic Review. Pract Radiat Oncol. 2020;10:423-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 121. | Rosenzweig KE, Zauderer MG, Laser B, Krug LM, Yorke E, Sima CS, Rimner A, Flores R, Rusch V. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2012;83:1278-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 122. | Rimner A, Spratt DE, Zauderer MG, Rosenzweig KE, Wu AJ, Foster A, Yorke ED, Adusumilli P, Rusch VW, Krug LM. Failure patterns after hemithoracic pleural intensity modulated radiation therapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2014;90:394-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 123. | Shaikh F, Zauderer MG, von Reibnitz D, Wu AJ, Yorke ED, Foster A, Shi W, Zhang Z, Adusumilli PS, Rosenzweig KE, Krug LM, Rusch VW, Rimner A. Improved Outcomes with Modern Lung-Sparing Trimodality Therapy in Patients with Malignant Pleural Mesothelioma. J Thorac Oncol. 2017;12:993-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 124. | Shaaban SG, Verma V, Choi JI, Shabason J, Sharma S, Glass E, Grover S, Badiyan SN, Simone CB 2nd. Utilization of Intensity-Modulated Radiation Therapy for Malignant Pleural Mesothelioma in the United States. Clin Lung Cancer. 2018;19:e685-e692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 125. | Scorsetti M, Bignardi M, Clivio A, Cozzi L, Fogliata A, Lattuada P, Mancosu P, Navarria P, Nicolini G, Urso G, Vanetti E, Vigorito S, Santoro A. Volumetric modulation arc radiotherapy compared with static gantry intensity-modulated radiotherapy for malignant pleural mesothelioma tumor: a feasibility study. Int J Radiat Oncol Biol Phys. 2010;77:942-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 126. | Dumane V, Yorke E, Rimner A, RosenzweigG K. SU-E-T-595: Comparison of Volumetric Modulated Arc Therapy (VMAT) and Static Intensity Modulated Radiotherapy (IMRT) for Malignant Pleural Mesothelioma in Patients with Intact Lungs/Post Pleurectomy. Med Phys. 2012;39:3842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Krayenbuehl J, Riesterer O, Graydon S, Dimmerling P, Kloeck S, Ciernik IF. Intensity-modulated radiotherapy and volumetric-modulated arc therapy for malignant pleural mesothelioma after extrapleural pleuropneumonectomy. J Appl Clin Med Phys. 2013;14:4130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 128. | Sterzing F, Sroka-Perez G, Schubert K, Münter MW, Thieke C, Huber P, Debus J, Herfarth KK. Evaluating target coverage and normal tissue sparing in the adjuvant radiotherapy of malignant pleural mesothelioma: helical tomotherapy compared with step-and-shoot IMRT. Radiother Oncol. 2008;86:251-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 129. | Kimura T, Doi Y, Nakashima T, Imano N, Katsuta T, Takahashi S, Kenjo M, Ozawa S, Murakami Y, Nagata Y. Clinical experience of volumetric modulated arc therapy for malignant pleural mesothelioma after extrapleural pneumonectomy. J Radiat Res. 2015;56:315-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 130. | Minatel E, Trovo M, Polesel J, Rumeileh IA, Baresic T, Bearz A, Del Conte A, Franchin G, Gobitti C, Drigo A, Dassie A, Pagan V, Trovo MG. Tomotherapy after pleurectomy/decortication or biopsy for malignant pleural mesothelioma allows the delivery of high dose of radiation in patients with intact lung. J Thorac Oncol. 2012;7:1862-1866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 131. | Parisi E, Romeo A, Sarnelli A, Ghigi G, Bellia SR, Neri E, Micheletti S, Dipalma B, Arpa D, Furini G, Burgio MA, Genestreti G, Gurioli C, Sanna S, Bovolato P, Rea F, Storme G, Scarpi E, Arienti C, Tesei A, Polico R. High dose irradiation after pleurectomy/decortication or biopsy for pleural mesothelioma treatment. Cancer Radiother. 2017;21:766-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 132. | Fodor A, Broggi S, Incerti E, Dell'Oca I, Fiorino C, Samanes Gajate AM, Pasetti M, Cattaneo MG, Passoni P, Gianolli L, Calandrino R, Picchio M, Di Muzio N. Moderately Hypofractionated Helical IMRT, FDG-PET/CT-guided, for Progressive Malignant Pleural Mesothelioma in Patients With Intact Lungs. Clin Lung Cancer. 2019;20:e29-e38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 133. | Pehlivan B, Sengul K, Yesil A, Nalbant N, Ozturk O, Ozdemir Y, Topkan E. Dosimetric Comparison of Lung-Sparing Radiation Therapy between Volumetric Arc Therapy and Helical Tomotherapy for Unresectable Malignant Pleural Mesothelioma. Biomed Res Int. 2019;2019:4568958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Schröder C, Opitz I, Guckenberger M, Stahel R, Weder W, Förster R, Andratschke N, Lauk O. Stereotactic Body Radiation Therapy (SBRT) as Salvage Therapy for Oligorecurrent Pleural Mesothelioma After Multi-Modality Therapy. Front Oncol. 2019;9:961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 135. | Krayenbuehl J, Hartmann M, Lomax AJ, Kloeck S, Hug EB, Ciernik IF. Proton therapy for malignant pleural mesothelioma after extrapleural pleuropneumonectomy. Int J Radiat Oncol Biol Phys. 2010;78:628-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Li YR, Alley EW, Friedberg JS, Culligan M, Busch TM, Hahn S, Cengel KA, Simone CB. Prospective assessment of proton therapy for malignant pleural mesothelioma. Paper presented at: 16th World Conference on Lung Cancer, 2015. [Cited in This Article: ] |
| 137. | Rice SR, Li YR, Busch TM, Kim MM, McNulty S, Dimofte A, Zhu TC, Cengel KA, Simone CB 2nd. A Novel Prospective Study Assessing the Combination of Photodynamic Therapy and Proton Radiation Therapy: Safety and Outcomes When Treating Malignant Pleural Mesothelioma. Photochem Photobiol. 2019;95:411-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 138. | Badiyan SN, Molitoris JK, Zhu M, Glass E, Diwanji T, Simone CB 2nd. Proton beam therapy for malignant pleural mesothelioma. Transl Lung Cancer Res. 2018;7:189-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 139. | Zeng J, Badiyan SN, Garces YI, Wong T, Zhang X, Simone CB 2nd, Chang JY, Knopf AC, Mori S, Iwata H, Meijers A, Li H, Bues M, Liu W, Schild SE, Rengan R; International Particle Therapy Cooperative Group Thoracic Subcommittee. Consensus Statement on Proton Therapy in Mesothelioma. Pract Radiat Oncol. 2021;11:119-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 140. | Kostron A, Friess M, Crameri O, Inci I, Schneiter D, Hillinger S, Stahel R, Weder W, Opitz I. Relapse pattern and second-line treatment following multimodality treatment for malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2016;49:1516-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 141. | Baldini EH, Richards WG, Gill RR, Goodman BM, Winfrey OK, Eisen HM, Mak RH, Chen AB, Kozono DE, Bueno R, Sugarbaker DJ. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2015;149:1374-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 142. | Soldera SV, Kavanagh J, Pintilie M, Leighl NB, de Perrot M, Cho J, Hope A, Feld R, Bradbury PA. Systemic Therapy Use and Outcomes After Relapse from Preoperative Radiation and Extrapleural Pneumonectomy for Malignant Pleural Mesothelioma. Oncologist. 2019;24:e510-e517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Opitz I, Scherpereel A, Berghmans T, Psallidas I, Glatzer M, Rigau D, Astoul P, Bölükbas S, Boyd J, Coolen J, De Bondt C, De Ruysscher D, Durieux V, Faivre-Finn C, Fennell DA, Galateau-Salle F, Greillier L, Hoda MA, Klepetko W, Lacourt A, McElnay P, Maskell NA, Mutti L, Pairon JC, Van Schil P, van Meerbeeck JP, Waller D, Weder W, Putora PM, Cardillo G. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2020;58:1-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 144. | Bibby AC, Tsim S, Kanellakis N, Ball H, Talbot DC, Blyth KG, Maskell NA, Psallidas I. Malignant pleural mesothelioma: an update on investigation, diagnosis and treatment. Eur Respir Rev. 2016;25:472-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |