Published online Apr 10, 2018. doi: 10.5306/wjco.v9.i2.33
Peer-review started: July 3, 2017
First decision: December 7, 2017
Revised: December 19, 2017
Accepted: February 5, 2018
Article in press: February 5, 2018
Published online: April 10, 2018
To investigate the survival impact of clinicopathological factors, including pathological complete response (pCR) and tumor-infiltrating lymphocytes (sTIL) levels according to subtypes, in breast cancer (BC) patients who received neo-adjuvant chemotherapy (NAC).
We evaluated 435 BC patients who presented and received NAC at the Instituto Nacional de Enfermedades Neoplasicas from 2003 to 2014. sTIL was analyzed as the proportion of tumor stroma occupied by lymphocytes, and was prospectively evaluated on hematoxylin and eosin-stained sections of the preNAC core biopsy. pCR was considered in the absence of infiltrating cancer cells in primary tumor and axillary lymph nodes. Analysis of statistical association between clinical pathological features, sTIL, pCR and survival were carried out using SPSSvs19.
Median age was 49 years (range 24-84 years) and the most frequent clinical stage was IIIB (58.3%). Luminal A, Luminal B, HER2-enriched and (triple-negative) TN phenotype was found in 24.6%, 37.9%, 17.7% and 19.8%, respectively. pCR was observed in 11% and median percentage of sTIL was 40% (2%-95%) in the whole population. pCR was associated to Ct1-2 (P = 0.045) and to high sTIL (P = 0.029) in the whole population. There was a slight trend towards significance for sTIL (P = 0.054) in Luminal A. sTIL was associated with grade III (P < 0.001), no-Luminal A subtype (P < 0.001), RE-negative (P < 0.001), PgR-negative (P < 0.001), HER2-positive (P = 0.002) and pCR (P = 0.029) in the whole population. Longer disease-free survival was associated with grade I-II (P = 0.006), cN0 (P < 0.001), clinical stage II (P = 0.004), ER-positive (P < 0.001), PgR-positive (P < 0.001), luminal A (P < 0.001) and pCR (P = 0.002). Longer disease-free survival was associated with grade I-II in Luminal A (P < 0.001), N0-1 in Luminal A (P = 0.045) and TNBC (P = 0.01), clinical stage II in Luminal A (P = 0.003) and TNBC (P = 0.038), and pCR in TNBC (P < 0.001). Longer overall survival was associated with grade I-II (P < 0.001), ER-positive (P < 0.001), PgR-positive (P < 0.001), Luminal A (P < 0.001), cN0 (P = 0.002) and pCR (P = 0.002) in the whole population. Overall survival was associated with clinical stage II (P = 0.017) in Luminal A, older age (P = 0.042) in Luminal B, and pCR in TNBC (P = 0.005).
Predictive and prognostic values of clinicopathological features, like pCR and sTIL, differ depending on the evaluated molecular subtype
Core tip: The authors evaluated a series of 435 breast cancer (BC) patients who received neoadjuvant chemotherapy. They evaluated the association between stromal tumor-infiltrating lymphocytes levels and pCR in preneoadjuvant chemotherapy samples according to molecular subtypes. The results confirm differences in the predictive and prognostic role of stromal tumor-infiltrating lymphocytes and pathological complete response depending on the tumor subtype. Additionally, the authors evaluate the value of traditional prognostic features in every BC subset. The results increase the understanding of biomarkers in the heterogeneous scenario of BC.
- Citation: Galvez M, Castaneda CA, Sanchez J, Castillo M, Rebaza LP, Calderon G, Cruz MDL, Cotrina JM, Abugattas J, Dunstan J, Guerra H, Mejia O, Gomez HL. Clinicopathological predictors of long-term benefit in breast cancer treated with neoadjuvant chemotherapy. World J Clin Oncol 2018; 9(2): 33-41
- URL: https://www.wjgnet.com/2218-4333/full/v9/i2/33.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i2.33
Breast cancer (BC) is the second most common cancer in the world and the most frequent cancer among women, with an estimated 1.67 million new cancer cases diagnosed in 2012 (25% of all cancers), and is the fifth cause of death from cancer overall (522000 deaths)[1]. Neoadjuvant chemotherapy (NAC) is the standard therapy for locally advanced BC and could improve both surgical options and long-term outcome[2]. Response to NAC is considered an in vivo test of tumor sensitivity to NAC, and the achievement of a pathological complete response (pCR) is associated with longer disease-free survival (DFS) and greater overall survival (OS)[3-7]. Tumor-infiltrating lymphocytes (TILs) serve to evaluate the host immune system response against a tumor and also constitutes a valuable predictive biomarker of NAC response and survival[8-11].
BC is a heterogeneous disease, and intrinsically different subtypes of BC have been identified in the past years based on gene expression profiles and on the combined immunohistochemical status of hormone and HER2 receptors. Responsiveness to preoperative therapies and outcome after surgery can be predicted by BC subtypes[12-14].
In this study, we investigated the survival impact of different clinicopathological factors, including pCR and TIL levels, according to the subtypes in BC patients who received NAC. The predictive role of different clinicopathological features for having high density TIL and obtaining pCR according to subtypes was also determined.
We found 435 patients diagnosed with BC at clinical stage IIB to IIIC at the Medical Department of the Instituto Nacional de Enfermedades Neoplasicas from 2003 to 2014. Eligibility criteria for this retrospective study were a histological diagnosis based on a core needle biopsy, having received NAC regimen and having undergone surgery after NAC. Patient characteristics such as age, clinical stage, histological subtype and grade, presence of estrogen receptors (ERs), progesterone receptors (PgRs) and HER2, and molecular subtype was obtained from the pathology report of preNAC core biopsy. pCR was defined as absence of invasive cancer in the breast and axillary nodes, irrespective of carcinoma in situ (ypT0/is ypN0), as previously described[4,15]. Phenotype classification was prospectively concluded through the evaluation of ER, PgR, HER2 and Ki67 as well as histological grade (in cases without Ki67 information): Luminal A (ER ≥ 10%, PgR ≥ 20%, HER2-negative and Ki67 < 15% or HG-I-II), Luminal B (ER ≥ 10% and any PgR < 20%, HER2-positive, Ki67 < 15% or HG-III), HER2-enriched (ER < 10%, PgR < 10% and HER2-positive) and triple-negative (TN) (ER < 10%, PgR < 10% and HER2-negative). Stromal (s)TIL was prospectively evaluated in preNAC core biopsy and was defined as percentage of stromal area covered by lymphocytes[16].
Follow-up and recurrence information (date and location) were obtained from patient files. Time-from-last-chemotherapy-to-surgery was considered as the number of months from the date of the last NAC administration to surgery of the primary tumor. OS was calculated from surgery date of the primary breast tumor to death or last follow-up date, and DFS was calculated from surgery date of the primary breast tumor to recurrence or last follow-up date.
Categorical comparisons and association analysis between clinical pathological features and pCR were carried out using the chi-square statistic or Fisher’s exact test. Survival analysis, regarding OS and DFS, was performed using the Kaplan-Meier method, and differences between curves were estimated by log-rank test. In all cases, the level of alpha was set at 0.05 a priori. Statistical analysis was performed using SPSS v19 (IBM Corp., Armonk, NY, United States).
There were 435 patients included in this study, with median age at diagnosis of 49 years (range: 24-84 years), median tumor size of 6.5 cm (range: 1.0-24.0 cm), T3 in 27.8% and T4 in 63.9%. Inflammatory disease was found in 29.2%. The most frequent clinical stages were IIIB (60.5%) and IIIA (18.6%). Ductal histology was found in 93.3%, high grade in 52.2%, ER+ status in 62.8%, PgR+ status in 51% and HER2+++ in 32.4%. Luminal A, Luminal B, HER2-enriched and TN phenotype was found in 24.6%, 37.9%, 17.7% and 19.8%, respectively. The most frequent NACs were doxorubicin-cyclophosphamide for 4 cycles followed by 12 weekly paclitaxel (67.18%), doxorubicin-cyclophosphamide for 4 cycles followed by every 3 wk paclitaxel in 4 cycles (18.85%) and doxorubicin-cyclophosphamide for 4 cycles alone (7.32%). The median time from the last chemotherapy to surgery was 63 d (maximum: 982 d). pCR was observed in 48 (11%) patients. Median percentage of sTILs was 40% (2%-95%) in the entire population and 70% (60%-95%) in patients with pCR. Recurrence was found in 35.7%. Median DFS was 7.54 and median OS was 5.16 years (95%CI: 4.16-6.15 years) (Table 1).
| Cases | sTIL ≥ 50% | P value | pCR | P value | Overall Survival at 5 yr(OS = 50.1%) | P value | Progression free survival at 5 yr(DFS = 57.8%) | P value | |
| 435 | 181 | 48 | |||||||
| Age (yr), median (range) | 49 (24-84) | 49 (24-84) | 0.923 | 47 (28-80) | 0.472 | 0.512 | 0.833 | ||
| < 50 | 231 (53.1) | 96 (35.2) | 28 (12.1) | 48.8% | 59.7% | ||||
| ≥ 50 | 204 (46.9) | 85 (36.7) | 20 (9.8) | 51.7% | 55.9% | ||||
| Histological subtypes | 0.928 | 0.234 | 0.512 | 0.497 | |||||
| Ductal | 406 (93.3) | 169 (43.6) | 43 (10.6) | 49.0% | 57.5% | ||||
| Lobular | 21 (4.8) | 7 (36.8) | 2 (9.5) | 61.0% | 55.2% | ||||
| Others | 8 (1.8) | 5 (62.5) | 3 (37.5) | - | - | ||||
| Histological grade | < 0.001 | 0.170 | 0.001 | 0.006 | |||||
| G1-G2 | 200 (46.0) | 59 (32.6) | 17 (8.5) | 57.1% | 64.6% | ||||
| G3 | 227 (52.2) | 119 (65.7) | 29 (12.8) | 42.8% | 52.2% | ||||
| NR | 8 (1.8) | 3 (1.7) | 2 (25) | 83.3% | 45.7% | ||||
| ER | < 0.001 | 0.098 | < 0.001 | 0.000 | |||||
| No | 162 (37.2) | 89 (57.8) | 23 (14.2) | 36.1% | 47.1% | ||||
| Yes | 273 (62.8) | 92 (35.2) | 25 (9.2) | 58.2% | 64.3% | ||||
| PgR | 0.003 | 0.246 | < 0.001 | 0.000 | |||||
| No | 213 (49) | 104 (51.0) | 27 (12.7) | 41.0% | 50.0% | ||||
| Yes | 222 (51) | 77 (36.5) | 21 (9.5) | 58.4% | 64.8% | ||||
| HER2 | 0.002 | 0.135 | 0.334 | 0.135 | |||||
| No | 294 (67.6) | 106 (38.3) | 28 (9.5) | 53.7% | 60.4% | ||||
| Yes | 141 (32.4) | 75 (54.3) | 20 (14.2) | 40.8% | 52.3% | ||||
| Molecular subtypes | < 0.001 | 0.233 | < 0.001 | < 0.001 | |||||
| Luminal A | 107 (24.6) | 30 (29.7) | 13 (12) | 72.0% | 76.1% | ||||
| Luminal B | 165 (37.9) | 61 (38.4) | 12 (7) | 50.6% | 57.7% | ||||
| HER2-enriched | 77 (17.7) | 50 (66.7) | 10 (13) | 41.5% | 54.9% | ||||
| Triple-Negative | 86 (19.8) | 40 (50.0) | 13 (15) | 32.5% | 40.3% | ||||
| Tumor size (cm) | 0.183 | 0.019 | 0.490 | 0.250 | |||||
| Median (range) | 6.5 (1-24) | 6.5 (1-16) | 6.0 (2-15) | ||||||
| cT | |||||||||
| cT1-cT2 | 36 (8.3) | 19 (54.3) | 8 (22.2) | 55.0% | 69.2% | ||||
| cT3-cT4 | 399 (91.7) | 162 (42.6) | 40 (10) | 49.6% | 56.8% | ||||
| cN | 0.084 | 0.743 | 0.007 | 0.001 | |||||
| cN0 | 83 (19.1) | 28 (35.0) | 10 (12) | 65.8% | 77.0% | ||||
| cN1-cN2-cN3 | 352 (80.9) | 153 (45.7) | 38 (10.8) | 47.2% | 54.2% | ||||
| Clinical stage | 0.192 | 0.088 | 0.155 | 0.004 | |||||
| II | 72 (16.6) | 26 (36.6) | 12 (16.7) | 62.1% | 74.3% | ||||
| III | 363 (83.4) | 155 (45.1) | 36 (9.9) | 48.1% | 55.4% | ||||
| sTIL% | 0.002 | 0.598 | 0.747 | ||||||
| Median (range) | 40 (2-95) | 70 (60-95) | 65 (5-95) | ||||||
| < 50% | 266 (61.1) | 0 (0) | 20 (7.5) | 49.6% | 55.7% | ||||
| ≥ 50% | 149 (34.3) | 181 (100) | 26 (17.4) | 53.9% | 63.1% | ||||
| Missing data | 20 (4.6) | 20 (0) | 2 (10) | - | - | ||||
| TLCS (d) | 0.411 | 0.633 | 0.317 | 0.156 | |||||
| Median (range) | 63 (5-982) | 58 (8-982) | 65 (8-281) | ||||||
| Shorter than median | 207 (47.6) | 91 (45.5) | 22 (10.6) | 48.5% | 55.0% | ||||
| Longer than median | 211 (48.5) | 82 (41.4) | 26 (12.3) | 56.7% | 61.2% | ||||
| Missing data | 17 (3.9) | 8 (47.1) | 0 (0) | 17.6% | 46.3% | ||||
| pCR | 0.029 | 0.002 | 0.002 | ||||||
| No | 387 (89) | 154 (41.7) | 0 (0) | 47.4% | 55.1% | ||||
| Yes | 48 (11) | 27 (58.7) | 48 (100) | 85.1% | 84.9% | ||||
| Relapse | 0.895 | < 0.001 | < 0.001 | ||||||
| No | 284 (65.3) | 118 (43.4) | 42 (14.8) | 81.6% | - | ||||
| Yes | 151 (34.7) | 63 (44.1) | 6 (4) | 8.58% | - |
Association analysis found that pCR was associated with T1-2 (P = 0.045) and to high sTIL level (P = 0.029) in the entire population (Table 1). Higher sTIL level had a slight trend towards association with pCR (P = 0.054) in Luminal A, and smaller tumor size had a trend towards association with pCR (P = 0.098) in Luminal A. Clinical involvement of axillary lymph nodes was not associated to variation of pCR (Table 2). An additional analysis by level of axillary involvement found that N2-3 had lower rates of pCR than N0-1 only in TNBC (P = 0.018).
| Lum A | Lum B | HER2 | TN | |||||||||
| Total | pCR | P value | Total | pCR | P value | Total | pCR | P value | Total | pCR | P value | |
| 107 | 13 | 165 | 12 | 77 | 10 | 86 | 13 | |||||
| Age (yr) | 1.000 | 0.315 | 0.507 | 0.157 | ||||||||
| median (range) | 47 (28-75) | 46 (28-62) | 51 (25-84) | 52 (39-69) | 51 (28-80) | 46 (29-80) | 49 (26-73) | 45 (28-68) | ||||
| < 50 | 72 (67) | 9 (13) | 78 (48) | 4 (5) | 37 (48) | 6 (16.2) | 44 (48) | 9 (20) | ||||
| ≥ 50 | 35 (33) | 4 (11) | 87 (52) | 8 (9) | 40 (52) | 4 (10) | 42 (52) | 4 (10) | ||||
| Histological subtypes | 0.349 | 1.000 | 0.434 | 0.392 | ||||||||
| Ductal | 97 (91) | 11 (11) | 153 (93) | 11 (7) | 73 (95) | 9 (12.3) | 83 (97) | 12 (14) | ||||
| Lobular and others | 10 (9) | 2 (20) | 12 (7) | 1 (8) | 4 (5) | 1 (25) | 3 (3) | 1 (33) | ||||
| Histological grade | - | 0.213 | 0.266 | 1.000 | ||||||||
| G1-G2 | 103 (97) | 12 (12) | 61 (39) | 2 (3) | 23 (30) | 1 (4.3) | 13 (15) | 2 (15) | ||||
| G3 | - | - | 102 (61) | 10 (10) | 53 (69) | 9 (17) | 72 (85) | 10 (14) | ||||
| NR | 4 (3) | 1 (25) | 2 (1) | 0 (0) | 1 (1) | 0 (0) | 1 (0) | 1 (100) | ||||
| Tumor size (cm) | 0.102 | 0.213 | 0.511 | 0.620 | ||||||||
| Median (range) | 6 (2-15) | 5 (2-9) | 7 (2-20) | 6 (2-12) | 7 (2.5-14) | 6 (4-12) | 7 (1-24) | 8 (3-15) | ||||
| cT1-cT2 | 10 (7) | 3 (30) | 12 (7) | 2 (17) | 5 (6) | 1 (20) | 9 (10) | 2 (22) | ||||
| cT3-cT4 | 97 (93) | 10 (10) | 153 (93) | 10 (7) | 72 (94) | 9 (12.5) | 77 (90) | 11 (14) | ||||
| cN | 0.306 | 0.222 | 0.270 | 0.021 | ||||||||
| cN0 | 27 (23) | 5 (19) | 28 (18) | 0 (0) | 53 (69) | 5 (9.4) | 14 (14) | 4 (29) | ||||
| cN1-cN2–cN3 | 80 (77) | 8 (10) | 137 (82) | 12 (9) | 24 (31) | 5 (20.8) | 72 (86) | 9 (13) | ||||
| Clinical stage | 0.471 | 0.652 | 1.000 | 0.122 | ||||||||
| EC II | 23 (20) | 4 (17) | 21 (12) | 2 (10) | 11 (14) | 1 (9.1) | 17 (16) | 5 (29) | ||||
| EC III | 84 (80) | 9 (11) | 144 (88) | 10 (7) | 66 (86) | 9 (13.6) | 69 (84) | 8 (12) | ||||
| sTIL% | 0.054 | 0.750 | 0.150 | 1.000 | ||||||||
| Median (range) | 30 (2-90) | 50 (10-90) | 40 (5-90) | 30 (8-90) | 60 (5-95) | 80 (30-95) | 45 (2-90) | 50 (5-80) | ||||
| < 50 | 71 (69) | 6 (8) | 98 (60) | 6 (6) | 25 (32) | 1 (4) | 40 (47) | 6 (15) | ||||
| ≥ 50 | 30 (24) | 7 (23) | 61 (37) | 5 (8) | 50 (66) | 9 (18) | 40 (47) | 6 (15) | ||||
| Missing data | 6 (6) | 0 (0) | 6 (3) | 1 (17) | 2 (3) | 0 (0) | 6 (7) | 1 (17) | ||||
| TLCS (d) | 0.233 | 0.238 | 0.744 | 0.500 | ||||||||
| Median (range) | 67 (14-458) | 80 (16-281) | 61 (5-412) | 54 (8-140) | 60 (11-240) | 66 (37-106) | 64 (8-982) | 66 (14-122) | ||||
| Shorter than median | 49 (48) | 4 (8) | 77 (45) | 8 (10) | 41 (53) | 5 (12.2) | 40 (48) | 5 (13) | ||||
| Longer than median | 57 (51) | 9 (16) | 76 (47) | 4 (5) | 33 (43) | 5 (15.2) | 45 (51) | 8 (18) | ||||
| Missing data | 1 (1) | 0 (0) | 12 (8) | 0 (0) | 3 (4) | 0 (0) | 1 (1) | 0 (0) | ||||
| Relapse | 0.121 | 0.753 | 0.300 | < 0.001 | ||||||||
| No | 87 (79) | 13 (15) | 109 (65) | 9 (8) | 46 (60) | 8 (17.4) | 42 (41) | 12 (29) | ||||
| Yes | 20 (21) | 0 (0) | 56 (35) | 3 (5) | 31 (40) | 2 (6.5) | 44 (59) | 1 (2) | ||||
Association analysis found that sTIL level was associated with grade III (P < 0.001), no-Luminal A subtype (P < 0.001), ER-negative (P < 0.001), PgR-negative (P < 0.001), HER2-positive (P = 0.002) and pCR (P = 0.029) in the entire population (Table 1). Within each BC subtype, sTIL level remained associated with grade III in Luminal B (P = 0.011) and TN (P = 0.006) subtypes, as well as cN+ in Luminal B (P = 0.02) (Table 3).
| Lum A | Lum B | HER2 | TN | |||||||||
| < 50% | ≥ 50% | P value | < 50% | ≥ 50% | P value | < 50% | ≥ 50% | P value | < 50% | ≥ 50% | P value | |
| 71 | 30 | 98 | 61 | 25 | 50 | 40 | 40 | |||||
| Age (yr) | 0.181 | 0.783 | 0.624 | 0.074 | ||||||||
| Median (range) | 47 (28-75) | 47 (36-74) | 52 (28-73) | 50 (25-84) | 52 (28-66) | 49 (29-80) | 51 (26-73) | 45 (27-73) | ||||
| < 50 | 50 (70) | 17 (57) | 46 (47) | 30 (49) | 11 (44) | 25 (50) | 16 (40) | 24 (60) | ||||
| ≥ 50 | 21 (30) | 13 (43) | 52 (53) | 31 (51) | 14 (56) | 25 (50) | 24 (60) | 16 (40) | ||||
| Histological subtypes | 0.445 | 1.000 | 0.597 | 1.000 | ||||||||
| Ductal | 66 (93) | 26 (87) | 91 (93) | 57 (93) | 23 (92) | 48 (96) | 39 (98) | 38 (95) | ||||
| Lobular and others | 5 (7) | 4 (13) | 7 (7) | 4 (7) | 2 (8) | 2 (4) | 1 (3) | 2 (5) | ||||
| Histological grade | - | 0.011 | 0.514 | 0.006 | ||||||||
| G1-G2 | 69 (97) | 28 (93) | 43 (44) | 15 (25) | 9 (36) | 14 (28) | 11 (28) | 2 (5) | ||||
| G3 | 0 (0) | 0 (0) | 53 (54) | 46 (75) | 16 (64) | 35 (71) | 29 (73) | 38 (95) | ||||
| NR | 2 (3) | 2 (7) | 2 (2) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | ||||
| Tumor size (cm) | ||||||||||||
| Median (range) | 6 (3-13) | 6 (2-15) | 6 (3-20) | 7 (2-15) | 7 (3-14) | 7 (3-14) | 7 (4-24) | 7 (1-16) | ||||
| cT | 1.000 | 0.538 | 0.659 | 0.263 | ||||||||
| cT1-cT2 | 7 (10) | 3 (10) | 6 (6) | 6 (10) | 1 (4) | 4 (8) | 2 (5) | 6 (15) | ||||
| cT3-cT4 | 64 (90) | 27 (90) | 92 (94) | 55 (90) | 24 (96) | 46 (92) | 38 (95) | 34 (85) | ||||
| cN | 0.890 | 0.020 | 0.631 | 0.762 | ||||||||
| cN0 | 18 (25) | 8 (27) | 22 (22) | 5 (8) | 6 (24) | 8 (16) | 6 (15) | 7 (18) | ||||
| cN1-cN2-cN3 | 53 (75) | 22 (73) | 76 (78) | 56 (92) | 11 (44) | 27 (54) | 34 (85) | 33 (83) | ||||
| Clinical Stage | 0.666 | 0.141 | 0.742 | 0.576 | ||||||||
| EC II | 17 (24) | 6 (20) | 16 (16) | 5 (8) | 3 (12) | 8 (16) | 9 (23) | 7 (18) | ||||
| EC III | 54 (76) | 24 (80) | 82 (84) | 56 (92) | 22 (88) | 42 (84) | 31 (78) | 33 (83) | ||||
| TLCS (d) | 0.631 | 0.882 | 0.502 | 0.141 | ||||||||
| Median (range) | 64 (14-449) | 70 (19-458) | 61 (5-412) | 58 (8-285) | 68 (16-234) | 56 (11-240) | 74 (24-230) | 51 (14-982) | ||||
| Shorter than median | 34 (48) | 13 (43) | 48 (49) | 28 (46) | 12 (48) | 28 (56) | 15 (38) | 22 (55) | ||||
| Longer than median | 36 (51) | 17 (57) | 44 (45) | 27 (44) | 12 (48) | 20 (40) | 24 (60) | 18 (45) | ||||
| Missing data | 1 (1) | 0 (0) | 6 (6) | 6 (10) | 1 (4) | 2 (4) | 1 (3) | 0 (0) | ||||
| pCR | 0.054 | 0.750 | 0.150 | 1.000 | ||||||||
| No | 65 (92) | 23 (77) | 92 (94) | 56 (92) | 24 (96) | 41 (82) | 34 (85) | 34 (85) | ||||
| Yes | 6 (8) | 7 (23) | 6 (6) | 5 (8) | 1 (4) | 9 (18) | 6 (15) | 6 (15) | ||||
| Relapse | 0.450 | 0.201 | 0.737 | 0.502 | ||||||||
| No | 59 (83) | 23 (77) | 61 (62) | 44 (72) | 16 (64) | 30 (60) | 18 (45) | 21 (53) | ||||
| Yes | 12 (17) | 7 (23) | 37 (38) | 17 (28) | 9 (36) | 20 (40) | 22 (55) | 19 (48) | ||||
Survival analysis found longer DFS was associated with grade I- II (P = 0.006), cN0 (P < 0.001), clinical stage II (P = 0.004), ER-positive (P < 0.001), PgR-positive (P < 0.001), Luminal A (P < 0.001) and pCR (P = 0.002). Longer DFS was associated with grade I- II in Luminal A (P = 0.033), N0-1 in Luminal A (P = 0.045) and TNBC (P = 0.01), clinical stage II in Luminal A (P = 0.003) and TNBC (P = 0.038), and pCR in TNBC (P = 0.001) (Table 1).
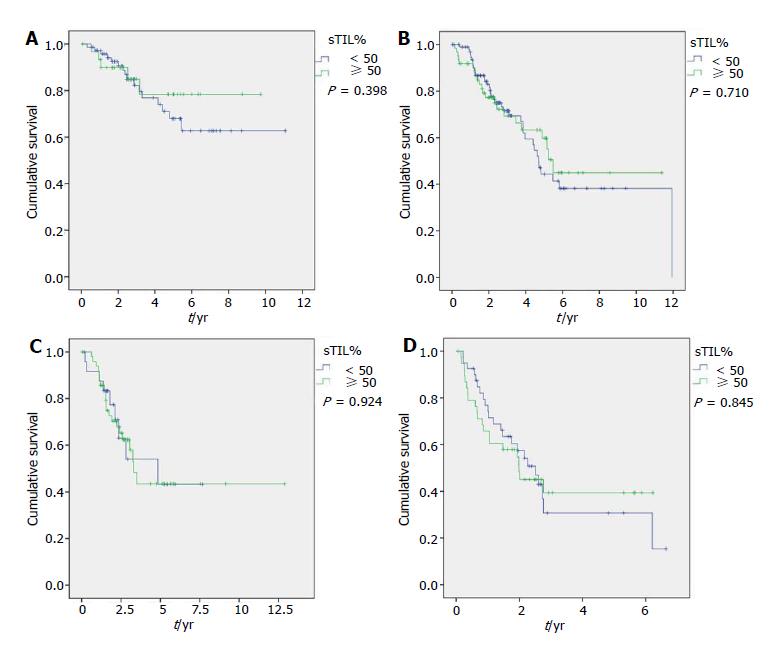
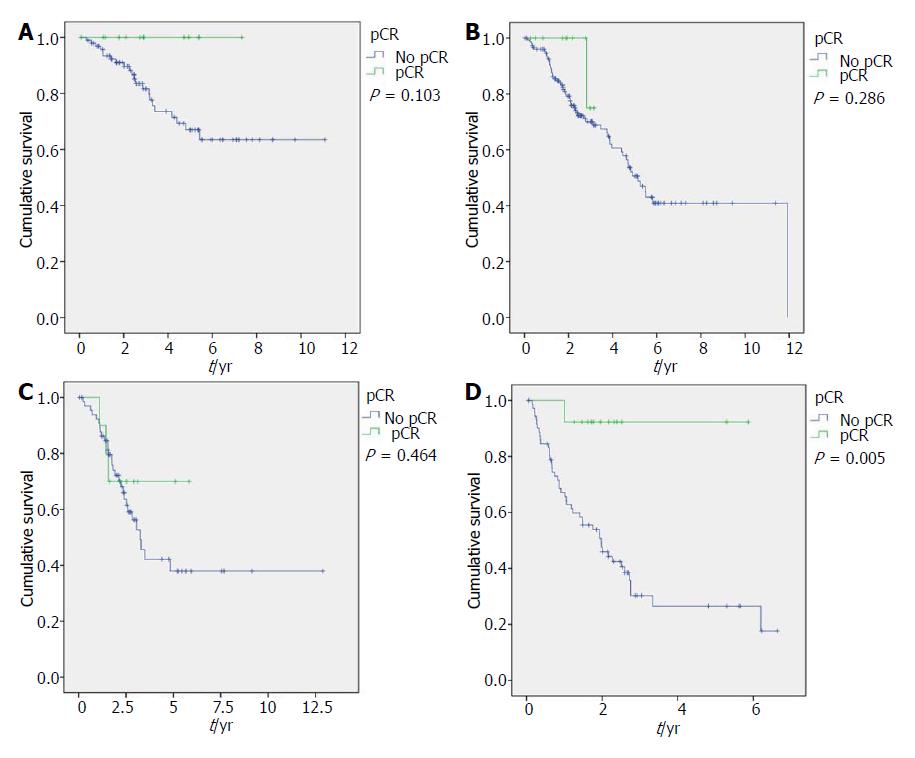
Longer OS was associated with grade I- II (P < 0.001), ER-positive (P < 0.001), PgR-positive (P < 0.001), Luminal A (P < 0.001), cN0 (P = 0.007) and pCR (P = 0.002) in the entire population. It was also associated with older age in Luminal B (P = 0.042), to clinical stage II in Luminal A (P = 0.017), and to cN0 (P = 0.045) and pCR in TNBC (P = 0.005) (Figure 1). Differences in TILs did not affect survival in the entire nor molecular subtype populations (Table 1 and Figure 2).
The biological heterogeneity of BC has been extensively described, and differences between intrinsic subtypes have been confirmed in the recent decade. We explored differences in the survival impact of tumor features, including pCR and TIL levels in each of the four molecular subtypes. Rates of pCR are lower in Luminal-A (9.2%), HER2-enriched (13%) and TNBC (15.3%) subtypes. pCR is also associated with longer survival in the entire population as well as in TNBC (pCR = 92.3% vs not pCR = 26.5% 5-year OS, P = 0.005; and trend in Luminal A, Luminal B and HER2-enriched phenotypic subsets of our series). It is widely assumed that patients who achieve pCR have significantly better DFS and OS rates in all molecular subtypes[12-14,17-19]. von Minckwitz et al[6] found pCR was not associated with prognosis only in Luminal A tumors in a series of 6377 patients with anthracycline-taxane-based NAC from 7 randomized trials; some authors claim it is related to the observed continuous tumor shrinkage occurring in their ER-positive tumor group during extended NAC, different than early and short-period tumor shrinkage observed in the ER-negative group[6,18-24].
pCR was more frequent in small tumors for both the entire population and the Luminal A subtype in our series. This finding is concordant with the previously mentioned idea that the effect of chemotherapy in Luminal A is slower than in other subtypes. Besides, Baron et al[18] found a similar lower rate of pCR in tumor size larger than 5 cm (P = 0.022) in their entire series (n = 608), but no association in the Luminal setting (P = 0.411). Higher grade of axillary involvement (cN2-3) was associated with lower rates of pCR only in the TNBC subset of our series. This lower response in bulky metastases could explain the previously described TNBC paradox phenomena of higher pCR rates but also higher distant relapse[21].
pCR was associated with higher percentage of sTILs in the entire population and also within the HER2-enriched subtype (P = 0.02). A trend towards association was found in Luminal A, Luminal B and TNBC. Different studies have found that high TIL levels in preNAC samples are associated to higher pCR rates in the entire BC population[25-27]. Wang et al[28] performed a meta-analysis with 23 studies including 13100 BC patients, and similarly found that high TIL level was associated with improved pCR rate in the entire population, and in HER2 and TNBC. A high TIL level significantly predicted longer OS in the entire population (P < 0.001) and in patients with HER2-positive (P = 0.005) BC and in TNBC patients (P < 0.001).
TIL showed association with grade III tumors in the entire population and in Luminal B and TNBC subsets in our series. Similarly, Pruneri et al[29] describes that higher TIL levels have a trend towards association with HG3 (P = 0.052) and was associated to Ki67 ≥ 50% (P < 0.0001) in a series of 897 TNBC cases, and could reflect the appearance of a larger amount of neoantigens that elicit an immunomediated response. Involvement of axillary lymph nodes was associated to higher TIL levels only in the Luminal B subset. High density of TILs has previously been described as associated to absence of lymph node involvement in the entire population of BC, and our results indicate that this association could differ by some subtypes[30]. Higher level of sTILs was not associated to longer survival in the entire population nor in any subtype in our series. This finding could be explained by the small size of our series and because the highest impact of TILs is over pCR instead of survival.
Our study has some limitations. First, because of the retrospective design of the study, different chemotherapy schemas were used depending on the oncologist decision and surgical election depending on surgeon. Second, the sample sizes of each BC subgroup are rather small, so the prognostic impact of every clinicopathological feature in each BC subtype should be investigated in a larger population in subsequent studies. Despite these limitations, this is the first comprehensive report of the NAC effect over breast molecular subtypes in a Latin-American population.
Breast cancer can be classified into Luminal A, Luminal B, HER2-enriched and triple-negative. Clinicopathological features can identify breast cancer prognosis and include pathological complete response (tumor sensibility to chemotherapy) and tumor-infiltrating lymphocytes (TILs; host activity against the tumor).
Discussion and new information about molecular breast cancer subtypes have been included in the most relevant cancer-related meeting, and more than 30,000 articles have been published in the last 2 years. Two biomarkers, pathological complete response (pCR) and TILs, have been re-defined and gained pathologist acceptance in the last 3 years.
The main objective is to evaluate the survival impact of different clinicopathological factors, including pCR and TIL levels, according to the subtypes in breast cancer patients who received neoadjuvant chemotherapy.
Evaluation of TIL levels was prospectively performed following international guidelines. Breast cancer cases were classified according to 2017 St Gallen Breast Cancer Meeting guidelines.
pCR was associated with cT1-2 (P = 0.045) and high stromal (s)TILs (P = 0.029) in the entire population. However, this relationship was not found for every molecular subtype, probably because of the small sample size. pCR was associated with longer disease-free survival in the entire population (P = 0.002) and in TNBC (P < 0.001), as well as to longer overall survival in the entire population (P = 0.002) and in TNBC (P = 0.005).
Predictive and prognostic value of clinicopathological features like pCR and sTIL level differ depending on the molecular subtype being evaluated. Identification of pCR and TIL roles in every molecular subtype will allow for identification of those patients who need more intense chemotherapy and those who will benefit from an immune-modulator treatment.
No information about the relevance of pCR and TILs in South-American women with breast cancer have been published in. An increase in the knowledge about prognosis impact of pCR and TIL in every molecular breast cancer subtype will allow for obtaining more effective personalized therapies. Furthermore, similar analysis needs to be done with more precise methods to evaluate response to chemotherapy and host immune activity, such as tumor residual burden and CD3/CD8 ratio, respectively.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Peru
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Cihan YB, Dirier A, Houvenaeghel G, Shao R, Vinh-Hung V S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Wang CH
| 1. | Carbognin L, Pilotto S, Nortilli R, Brunelli M, Nottegar A, Sperduti I, Giannarelli D, Bria E, Tortora G. Predictive and Prognostic Role of Tumor-Infiltrating Lymphocytes for Early Breast Cancer According to Disease Subtypes: Sensitivity Analysis of Randomized Trials in Adjuvant and Neoadjuvant Setting. Oncologist. 2016;21:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Vila J, Mittendorf EA, Farante G, Bassett RL, Veronesi P, Galimberti V, Peradze N, Stauder MC, Chavez-MacGregor M, Litton JF. Nomograms for Predicting Axillary Response to Neoadjuvant Chemotherapy in Clinically Node-Positive Patients with Breast Cancer. Ann Surg Oncol. 2016;23:3501-3509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Issa-Nummer Y, Loibl S, von Minckwitz G, Denkert C. Tumor-infiltrating lymphocytes in breast cancer: A new predictor for responses to therapy. Oncoimmunology. 2014;3:e27926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2456] [Cited by in F6Publishing: 2725] [Article Influence: 272.5] [Reference Citation Analysis (2)] |
| 5. | Bossuyt V, Provenzano E, Symmans WF, Boughey JC, Coles C, Curigliano G, Dixon JM, Esserman LJ, Fastner G, Kuehn T, Peintinger F, von Minckwitz G, White J, Yang W, Badve S, Denkert C, MacGrogan G, Penault-Llorca F, Viale G, Cameron D; Breast International Group-North American Breast Cancer Group (BIG-NABCG) collaboration. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol. 2015;26:1280-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796-1804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1539] [Cited by in F6Publishing: 1723] [Article Influence: 143.6] [Reference Citation Analysis (0)] |
| 7. | Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1238] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 8. | Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macrì L, Daniele L, Oliaro A. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365-371; discussion 371-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, Darcy PK, Loi S. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13:202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 10. | Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology. 2013;2:e24720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016;2:1354-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 366] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 12. | Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA. Molecular portraits of human breast tumours. Nature. 2000;406:747-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10665] [Cited by in F6Publishing: 10416] [Article Influence: 434.0] [Reference Citation Analysis (0)] |
| 13. | Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678-5685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1316] [Cited by in F6Publishing: 1297] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 14. | Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 414] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 15. | Pennisi A, Kieber-Emmons T, Makhoul I, Hutchins L. Relevance of Pathological Complete Response after Neoadjuvant Therapy for Breast Cancer. Breast Cancer (Auckl). 2016;10:103-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1473] [Cited by in F6Publishing: 1856] [Article Influence: 185.6] [Reference Citation Analysis (0)] |
| 17. | Colleoni M, Bagnardi V, Rotmensz N, Dellapasqua S, Viale G, Pruneri G, Veronesi P, Torrisi R, Luini A, Intra M. A risk score to predict disease-free survival in patients not achieving a pathological complete remission after preoperative chemotherapy for breast cancer. Ann Oncol. 2009;20:1178-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Baron P, Beitsch P, Boselli D, Symanowski J, Pellicane JV, Beatty J, Richards P, Mislowsky A, Nash C, Lee LA. Impact of Tumor Size on Probability of Pathologic Complete Response After Neoadjuvant Chemotherapy. Ann Surg Oncol. 2016;23:1522-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, Walls A, Bousamra A, Ramineni M, Sinn B. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol. 2017;35:1049-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 415] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 20. | Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, Buchholz T, Meric F, Middleton L, Hortobagyi GN. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 21. | Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329-2334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1438] [Cited by in F6Publishing: 1485] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 22. | Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165-4174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 852] [Cited by in F6Publishing: 816] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 23. | Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672-2685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1625] [Cited by in F6Publishing: 1545] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 24. | Moon HG, Im SA, Han W, Oh DY, Han SW, Keam B, Park IA, Chang JM, Moon WK, Cho N. Estrogen receptor status confers a distinct pattern of response to neoadjuvant chemotherapy: implications for optimal durations of therapy: distinct patterns of response according to ER expression. Breast Cancer Res Treat. 2012;134:1133-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0152500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 26. | Krishnamurti U, Wetherilt CS, Yang J, Peng L, Li X. Tumor-infiltrating lymphocytes are significantly associated with better overall survival and disease-free survival in triple-negative but not estrogen receptor-positive breast cancers. Hum Pathol. 2017;64:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, Kiermaier A, Swain SM, Baselga J, Michiels S. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18:52-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 28. | Wang K, Xu J, Zhang T, Xue D. Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: A meta-analysis. Oncotarget. 2016;7:44288-44298. [PubMed] [Cited in This Article: ] |
| 29. | Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, Colleoni AM, Goldhirsch A, Viale G. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 30. | Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959-2966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 783] [Cited by in F6Publishing: 910] [Article Influence: 101.1] [Reference Citation Analysis (0)] |