Non-random host tree infestation by the Neotropical liana Marcgravia longifolia
- Published
- Accepted
- Received
- Academic Editor
- Todd Vision
- Subject Areas
- Ecology, Plant Science
- Keywords
- Tropical ecology, Amazonia, Liana, Marcgraviaceae, Host infestation, Lecythidaceae, Bark shedding
- Copyright
- © 2022 Heymann et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Non-random host tree infestation by the Neotropical liana Marcgravia longifolia. PeerJ 10:e14535 https://doi.org/10.7717/peerj.14535
Abstract
The question whether or not tropical lianas infest host trees randomly or they exert host selection has implications for the structure and dynamics of tropical rainforests, particularly if colonization by lianas impacts host fitness. In this study, we present evidence that the Neotropical liana Marcgravia longifolia (Marcgraviaceae) infests host trees non-randomly. We identified host trees to species or genus level for 87 of the 100 M. longifolia individuals found in the study area of the Estación Biológica Quebrada Blanco (EBQB) in north-eastern Peruvian Amazonia. Data on host availability were taken from two 1-ha plots sampled at EBQB as part of a large-scale tree inventory in western Amazonia. Of the total of 88 tree genera with two or more individuals present in the inventory, 18 were represented amongst hosts. Host genera with a probability of colonization higher than expected by chance were Eschweilera (Lecythidaceae), Pouteria (Sapotaceae), Brosimum (Moraceae), and Hymenaea (Fabaceae). These findings suggest that M. longifolia exerts some level of host selectivity, but the mechanisms for this are completely unknown. Given the large number of animal species (41 bird species, three primate species) that are dispersing the seeds of M. longifolia and that have diverse ecological strategies, directed seed dispersal is unlikely to account for the observed patterns of host infestation.
Introduction
Negative impacts of liana infestation on tree growth, reproduction and survival, and alteration of forest dynamics may significantly decrease the carbon storage potential of tropical ecosystems (García León et al., 2018; Ingwell et al., 2010; Kainer et al., 2006; Laurance et al., 2014; Schnitzer & Bongers, 2011; van der Heijden et al., 2013). Despite their ecological importance, the ecology of lianas has been much less studied than that of trees, although considerable progress has been made in recent years (Schnitzer, Mangan & Hubbell, 2015). An important aspect for forecasting future impacts on forest dynamics is the question of whether or not lianas prefer or avoid specific host tree species.
Infestation patterns have been mainly addressed from the perspective of trees, i.e., which proportion of the tree community is infested and how severe infestations are. Fast-growing tree species that are tall as adults are less likely to support lianas (van der Heijden, Healey & Phillips, 2008; Sfair et al., 2016), and light-demanding tree species have lower liana prevalence (Visser et al., 2018). Trees with larger diameter at breast height (dbh) support more lianas and a higher liana biomass (Pérez-Salicrup & de Meijere, 2005). In some studies, host tree traits such as bark roughness and flakiness, emerged as factors influencing infestation (van der Heijden, Healey & Phillips, 2008; Carsten et al., 2002), while in other studies factors related to light and dispersal seemed to be more important than host tree traits (Malizia & Grau, 2006). Finally, there are habitat differences (tropical rainforest, savanna woodland, tropical semi-deciduous forest) in the interaction between lianas and hosts, possibly structured by morphological traits (e.g., bark characteristics) of host trees (Zulqarnain Silva et al., 2016). However, the question whether lianas prefer or avoid certain tree species as hosts has rarely been addressed. In an Amazonian forest some tree species were less infested than would be expected from the mean percent of infestation (Pérez-Salicrup, Sork & Putz, 2001). In a study in the Indian Eastern Ghats, a few tree species were more strongly infested than expected from their abundance (Chittibabu & Parthasarathy, 2001), and in northern temperate forests some liana species seem to preferentially infest certain tree species (Leicht-Young et al., 2010). In regenerating deciduous forests in the Piedmont region of New Jersey (USA), four of the five most abundant lianas preferred early successional trees (Ladwig & Meiners, 2010). Finally, in regenerating lowland rainforest in Ile-Ife (Nigeria) three liana species preferentially infested specific tree species (Uwalaka, Borisade & Rufai, 2021).
In this article, we examine liana-host tree associations from the perspective of the liana, i.e., we test whether a specific liana species infests hosts according to their availability or whether there are host preferences and avoidances, respectively. Our model system is the woody liana Marcgravia longifolia from the Neotropical family Marcgraviaceae. Marcgravia longifolia is unusual within the family by presenting long pedunculate and flagelliflorous cauligenous inflorescences and infructescences that arise from the unbranched stem from ground level up to the canopy (Fig. 1A). Nectaries attract bats and hummingbirds, and a large number of frugivorous animals (birds, primates, and bats) feed on the fruits and disperse the seeds (Tirado Herrera et al., 2003; Domingues Paciência, 2014; Willems, 2016). Marcgravia longifolia individuals are rooted close to the base of their host trees; juveniles creep up trunks and attach by adventitious roots (Heald, de Roon & Dressler, 2002). During our research on the plant-animal interactions of M. longifolia, we got the impression that this liana is found more frequently on some host trees than on others. Therefore, we set out to systematically test the hypothesis that M. longifolia infests certain host tree species more frequently than would be expected from tree availability against the null hypothesis that infestation is random.
Figure 1: Growth habit of Marcgravia longifolia.
(A) The cauligenous infructescences of Marcgravia longifolia are present all along the trunk from ground level to canopy level. (B) A Marcgravia longifolia individual (red arrow) growing on the trunk of an Eschweilera coriacea tree with strongly exfoliating bark.Methods
Study site
The study was conducted from October 2013 to January 2014 at the Estación Biologica Quebrada Blanco (EBQB), located in north-eastern Peruvian Amazonia (4°21′S, 73°09′W), 70 km southeast of Iquitos. Primary terra-firme forest (“bosque de altura”; Encarnación, 1985) interspersed with small swampy areas (“bajiales”; Encarnación, 1985) dominates the 100 ha study area which is equipped with a trail system with 100 m ×100 m grids. Annual rainfall is around 3000 mm with a peak from February to May and a trough between August and October. For further details of EBQB see Heymann & Tirado Herrera (2021) and Heymann, Tirado Herrera & Dolotovskaya (2021).
Data collection
Since M. longifolia is a fruit resource for several primate species (Tirado Herrera et al., 2003), many M. longifolia-individuals (and their GPS position) were known from continuous primate ecological field work at EBQB. To detect additional individuals, we employed two strategies: (1) Systematic transect walks along all trails of the trail system throughout the study area. This allowed detection of reproductive M. longifolia-individuals within 25-30 m from the trails. (2) Systematic search with in the 1 ha-cells of the trail grid system, to detect individuals not visible from the trails. In combination, this resulted in a full inventory of reproductive M. longifolia-individuals in the study area. For all individuals the GPS position was recorded. Detection of M. longifolia is facilitated by their unique growth habit with inflorescences and infructescences protruding from the trunk (Fig. 1A) which can be seen from a long distance, particularly during the fruiting period. We identified M. longifolia host trees using a regional flora (Vásquez Martínez, 1997). All except one M. longifolia individual (found in a “bajial”) were growing in terra-firme forest (“bosque de altura”) with a flat or only slightly undulating terrain (“bosque de terraza”; Encarnación, 1985). No M. longifolia individuals were found in those parts of the study area with strongly undulating terrain (“bosque de colina”; Encarnación, 1985). All field work was performed under permits from the Peruvian Ministry of Agriculture in Lima (permits 0073-2014-MINAGRI-DGFFS/DGEFFS, 304-2018-MINAGRI-SERFOR-DGGSPFFS, 445-2018-MINAGRI-SERFOR-DGGSPFFS, 528-2019-MINAGRI-SERFOR-DGGSPFFS).
Data analyses
To estimate the extent to which M. longifolia selected host tree species randomly, i.e., according to availability, or preferred/avoided certain species, respectively, and the extent to which such potential preferences differed between genera, we first compiled a list of tree species and their abundance at EBQB. Data were taken from an inventory of trees >10 cm dbh in two 1-ha plots (a total of 1,087 trees) by Dávila Cardozo & Ríos Paredes (2006) which was part of a large-scale study on Amazonian tree communities (Pitman et al., 2008), (ter Steege et al., 2013). These two plots are located in terra-firme forest (“bosque de altura”) of the “bosque de terraza”-type (Encarnación, 1985), i.e., more or less flat terrain, and without swampy areas; thus, they are representative for the type of habitat where we found M. longifolia. As the number of tree species (294 species; Dávila Cardozo & Ríos Paredes, 2006) was much larger than the number of M. longifolia individuals and to minimize potential errors introduced through plant identifications by different botanists (from the inventory and the present study), we aggregated the tree list to genera. We excluded all genera that were represented by only one individual in the inventory from the analyses and four individuals from two different species that could not be identified to genus level. The number of individuals per genus in the inventory (2 ha) was linearly extrapolated to the 78.3 ha area over which M. longifolia were found in the EBQB study area, calculated as a Minimum Convex Polygon around the GPS locations. The resulting number of individuals per genus was then used for further analyses (see y-axis labels in Fig. 2).
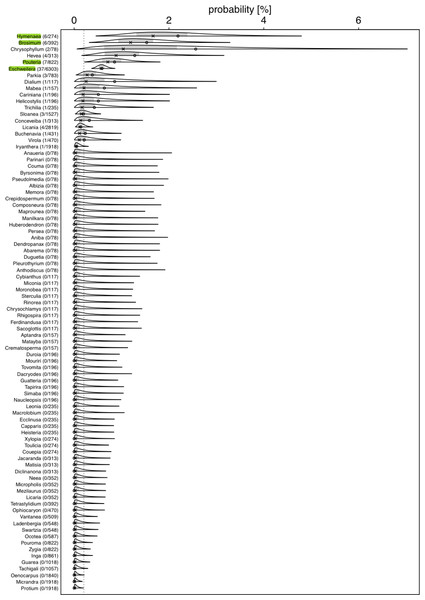
Figure 2: Posterior density for probability (in %) of a tree hosting a Marcgravia longifolia across tree genera.
Numbers after genus names indicate number of infested trees per genus / extrapolated number of tree individuals per genus. Laying cross: posterior median; open dot: observed infestation probability; grey area: central 50% probability interval; plotting limits of densities: central 99% probability interval (all referring to the genera specific posterior distributions). Highlighted genera are those where the 99% credible intervals does not overlap with the overall probability of a tree being infestedWe used a Bayesian hierarchical regression model (Gelman, Hill & Yajima, 2012) for estimating the probability (Bernoulli distributed response, Logit link function; McCullagh & Nelder, 1989) of a tree hosting the liana, conditional on the genus of the tree (i.e., genus was included as a grouping variable or ‘random intercepts effect’; Gelman & Hill, 2006). We used the R statistical software environment (version 4.1.1; R Core Team, 2022) and especially the ’Stan’ based package ‘brms’ (version 2.15.0; Bürkner, 2017; Bürkner, 2018; Carpenter et al., 2017) to conduct this estimation. As recommended by the Stan Developers (2017), we used a truncated (at 0) Student-t distribution with 4 degrees of freedom as prior for the standard deviation of the hierarchically modelled differences (on the logit scale) between the genus-specific expected values. The Markov Chain Monte Carlo simulation of the posterior—as performed by brms—was run for 10 Markov chains, each with 5,000 iterations and a burn-in period of 2,500 iterations, and we set the Stan control parameters adapt_delta and max_treedepth to 0.95 and 20, respectively. The full R code reproducing our results is provided as Supplementary Material. As an overall test of whether genera differed in their probabilities to host a liana we inspected the 99% highest posterior density interval (determined using the function hdi of the package HDInterval, version 0.2.2; Meredith & Kruschke, 2020) of the estimated standard deviation (in link space) for the variability among genera. If this does not include the value of 0, the effect of genus on the probability of hosting a liana has a 0.99 posterior probability—given our data and model—to be larger than zero. The response was not overdispersed given the model (dispersion parameter assessed from an equivalent Generalized Linear Mixed Model: 0.101).
Results
During research on plant-animal interactions of M. longifolia and during studies on the ecology of small New World monkeys, we found a total of 100 adult M. longifolia individuals at our study site in northeaster Peruvian Amazonia. For 87 M. longifolia individuals, the host tree could be identified to species or genus level. Of the total of 88 tree genera with two or more individuals present in the tree inventory (≥10 cm dbh) of Dávila Cardozo & Ríos Paredes (2006), trees from 18 genera (20.5% of genera) were hosts; five other host genera were not represented in the inventory (Table S1). With 37 infested individuals, Eschweilera (Lecythidaceae) was the genus with the largest number of infested tree individuals; only one to seven individuals of other host genera were infested by M. longifolia (Table S1). Within Eschweilera 26 individuals (70%) belonged to Eschweilera coriacea, while the remainder belonged to three other species.
For several genera, the estimated probabilities of being infested were high and reached values of up to 1.66% (posterior median; Fig. 2). However, the 99% credible intervals for most of them were fairly wide. For Eschweilera (Lecythidaceae), Pouteria (Sapotaceae), Brosimum (Moraceae), and Hymenaea (Fabaceae), the credible intervals did not overlap with the overall probability of a tree being infested (Fig. 2; Table S2); also, for Eschweilera the credible interval was very narrow compared to other genera. For the majority of genera, infestation probability was estimated to be close to zero and associated with low uncertainly (i.e., narrow credible intervals). The 99% credible interval for the variability (standard deviation of estimated genera specific deviations of their logit transformed infestation probabilities from the estimated logit transformed average infestation probability) among genera was estimated to range from 1.04 to 3.15 which is equivalent to an overall significant variation among genera with regard to their infestation probability (in case infestation probability would be similar in all species, particularly the lower but also the upper limit of this credible would be considerably closer to zero).
Discussion
Our results revealed a clear non-random component in the process that generates the infestation of hosts by M. longifolia. The estimated probability that a tree was infested varied considerably among genera. It was well above expected infestation probability in some and essentially zero in others. Although Eschweilera is the most common tree genus at our study site, it is infested more than would be expected from availability; trees from the second most common genus, Licania, are only very rarely infected. This strongly suggests that host choice may occur in M. longifolia.
The high probability of infestation of Eschweilera is surprising. It has been established that bark properties are influencing the probability of liana infestation: tree species with strongly exfoliating bark (flakiness) usually present lower levels of liana infestation since continuous debarking prevents lianas from growing successfully (Carsten et al., 2002; Carrasco-Urra & Gianoli, 2009; Jiménez-Castillo & Lusk, 2009). Similarly, trees with a smooth bark lack attachment points for climbers to grow on (Carsten et al., 2002; Balfour & Bond, 1993; Campbell & Newbery, 1993; Putz, 1980; Putz, 1984). Bark properties of Eschweilera trees vary between species (Mori, Black & de Zeeuw, 1987). The bark of Eschweilera coriacea (the most common Eschweilera species at EBQB with 68% of individuals of the genus) is strongly exfoliating (Fig. 1B), while the bark of other Eschweilera species is smooth, particularly in juvenile individuals (Ribeiro et al., 1999). These bark properties represent a challenge for liana infestation, but we do not know the strategy with which M. longifolia overcomes this challenge.
Since M. longifolia individuals are rooted close to the base of their host trees, seeds have to germinate next to the tree base to successfully infest a tree and grow up the trunk. Thus, the biased distribution of infestations over tree species could be a consequence of seed dispersal vectors of M. longifolia preferentially visiting Eschweilera trees for feeding, roosting, or sleeping. Fruits of M. longifolia are consumed by at least 41 species of birds, three species of primates, and >10 species of bats (Tirado Herrera et al., 2003; Domingues Paciência, 2014; Willems, 2016; Gottstein, 2018; Thiel, 2021). With the exception of coppery titi monkeys, Plecturocebus cupreus, none of these species is likely to exploit the hard-husked fruits of Eschweilera. Furthermore, M. longifolia and Eschweilera exhibit only a small overlap in their fruiting phenology: late “dry” to early rainy season in the former, and almost the entire rainy season in the latter (Tirado Herrera et al., 2003; Flores et al., 2015). Therefore, it is unlikely that seed dispersing frugivores—by targeting Eschweilera trees for feeding—disperse M. longifolia seeds selectively to the base of Eschweilera trunks. Alternatively, seed dispersal to Eschweilera trunks could occur if these trees were used for resting and sleeping. This can be excluded for the three primate species (Leontocebus nigrifrons, Saguinus mystax, Plecturocebus cupreus) whose sleeping and resting habits are well known (Heymann, 1995; Smith et al., 2007; Muñoz Lazo et al., 2011; Heymann et al. unpublished data); Eschweilera trees are not or very rarely used for sleeping and resting, respectively. In contrast to primates, sleeping and resting habits are largely unknown for the bird and bat species feeding on M. longifolia fruits, and thus they cannot be excluded as a factor creating directed seed dispersal to Eschweilera trees. However, we consider it more likely that the large and diverse spectrum of seed-dispersing frugivores (birds, bats, primates) exploiting M. longifolia fruits creates a wide seed shadow, and that through unknown mechanisms M. longifolia is selectively recruited at and/or infesting Eschweilera trees.
In summary, our study provides tentative evidence for non-random host infestation in the Neotropical liana M. longifolia. It adds to a little-studied aspect of liana ecology and raises further questions on the mechanisms that may result in host choice by lianas and stimulate further studies, which include possible mechanisms of host selectivity.
Conclusions
Our study provides preliminary evidence for non-random host infestation in the Neotropical liana M. longifolia and adds to the sparse literature on host selectivity in lianas. Future studies must identify the mechanisms of non-random host infestation, including traits of the liana and the hosts and properties of the microhabitat (e.g., soils) around the hosts.
Supplemental Information
Host and non-host tree genera
The tree genera that host (in bold) or do not host Marcgravia longifolia. It provides the the number and percentage of tree individuals per genus, the number of infested individuals, and the percentage of the respective tree genus amongst all tree infested by Marcgravia longifolia. The last column includes the number of individuals per genus extrapolated from the survey area (2 ha) to the area over which Marcgravia longifolia is distributed in the EBQB study area (78.3 ha).
Estimated species specific infestation probabilities (inverse logit transformed estimated Best Linear Unbiased Predictors plus the intercept)
CI: credible interval