Abstract
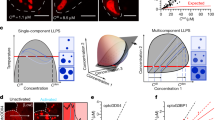
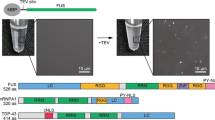
Liquid-liquid phase separation (LLPS) underlies the formation of biomolecular condensates, i.e., membrane-less compartments in cells that carry out functions related to RNA metabolism, stress adaptation, transport, or signaling. Examples of such biomolecular condensates are the nucleolus, nuclear speckles, promyelocytic leukemia protein (PML) bodies and paraspeckles in the nucleus, and stress granules and P bodies in the cytoplasm. Other structures in cells that are not typically viewed as bona fide compartments also seem to be formed via LLPS as recently elucidated, including heterochromatin, super-enhancers, and membrane receptor clusters. Key protein and/or RNA components of these biomolecular condensates form a scaffold via LLPS. Other constituents incorporate into this scaffold as clients. To understand the sequence features and interactions that mediate biomolecular condensate formation in cells, it is useful to quantify phase separation of pure components in vitro. Microscopy and turbidity measurements can be used to determine the concentration of a protein above which it phase separates, the so-called saturation concentration. Here, we describe experiments for the determination of full coexistence lines of phase-separating proteins by centrifugation. Coexistence lines are reconstructed from coexisting light and dense phase concentrations of the protein, and we present them as so-called phase diagrams. Phase diagrams allow the quantitative comparison of phase separation for proteins and their mutants under different conditions. They are thus important for our nuanced understanding of the driving forces underlying liquid-liquid phase separation in vitro. Such results have direct applicability for understanding phase separation-driven compartmentalization of cells.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Brangwynne CP, Eckmann CR, Courson DS et al (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324(5935):1729–1732. https://doi.org/10.1126/science.1172046
Brangwynne CP, Mitchison TJ, Hyman AA (2011) Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci U S A 108(11):4334–4339. https://doi.org/10.1073/pnas.1017150108
Nott TJ, Petsalaki E, Farber P et al (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell 57(5):936–947. https://doi.org/10.1016/j.molcel.2015.01.013
Molliex A, Temirov J, Lee J et al (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163(1):123–133. https://doi.org/10.1016/j.cell.2015.09.015
Patel A, Lee HO, Jawerth L et al (2015) A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162(5):1066–1077. https://doi.org/10.1016/j.cell.2015.07.047
Elbaum-Garfinkle S, Kim Y, Szczepaniak K et al (2015) The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc Natl Acad Sci U S A 112(23):7189–7194. https://doi.org/10.1073/pnas.1504822112
Zhang H, Elbaum-Garfinkle S, Langdon EM et al (2015) RNA controls PolyQ protein phase transitions. Mol Cell 60(2):220–230. https://doi.org/10.1016/j.molcel.2015.09.017
Lin Y, Protter DS, Rosen MK et al (2015) Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol Cell 60(2):208–219. https://doi.org/10.1016/j.molcel.2015.08.018
Berry J, Weber SC, Vaidya N et al (2015) RNA transcription modulates phase transition-driven nuclear body assembly. Proc Natl Acad Sci U S A 112(38):E5237–E5245. https://doi.org/10.1073/pnas.1509317112
Bracha D, Walls MT, Wei MT et al (2018) Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds. Cell 175(6):1467–1480. https://doi.org/10.1016/j.cell.2018.10.048. e1413
Hyman AA, Weber CA, Julicher F (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30:39–58. https://doi.org/10.1146/annurev-cellbio-100913-013325
Rai AK, Chen JX, Selbach M et al (2018) Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 559(7713):211–216. https://doi.org/10.1038/s41586-018-0279-8
Banani SF, Lee HO, Hyman AA et al (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18(5):285–298. https://doi.org/10.1038/nrm.2017.7
Mittag T, Parker R (2018) Multiple modes of protein-protein interactions promote RNP granule assembly. J Mol Biol 430(23):4636–4649. https://doi.org/10.1016/j.jmb.2018.08.005
Shin Y, Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease. Science 357(6357). https://doi.org/10.1126/science.aaf4382
Burke KA, Janke AM, Rhine CL et al (2015) Residue-by-residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol Cell 60(2):231–241. https://doi.org/10.1016/j.molcel.2015.09.006
Murakami T, Qamar S, Lin JQ et al (2015) ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88(4):678–690. https://doi.org/10.1016/j.neuron.2015.10.030
Conicella AE, Zerze GH, Mittal J et al (2016) ALS mutations disrupt phase separation mediated by alpha-helical structure in the TDP-43 low-complexity C-terminal domain. Structure 24(9):1537–1549. https://doi.org/10.1016/j.str.2016.07.007
Brady JP, Farber PJ, Sekhar A et al (2017) Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc Natl Acad Sci U S A 114(39):E8194–E8203. https://doi.org/10.1073/pnas.1706197114
Alberti S, Saha S, Woodruff JB et al (2018) A user’s guide for phase separation assays with purified proteins. J Mol Biol 430(23):4806–4820. https://doi.org/10.1016/j.jmb.2018.06.038
Ceballos AV, McDonald CJ, Elbaum-Garfinkle S (2018) Methods and strategies to quantify phase separation of disordered proteins. Methods Enzymol 611:31–50. https://doi.org/10.1016/bs.mie.2018.09.037
Mitrea DM, Chandra B, Ferrolino MC et al (2018) Methods for physical characterization of phase-separated bodies and membrane-less organelles. J Mol Biol 430(23):4773–4805. https://doi.org/10.1016/j.jmb.2018.07.006
Alberti S, Gladfelter A, Mittag T (2019) Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176(3):419–434. https://doi.org/10.1016/j.cell.2018.12.035
Mackenzie IR, Nicholson AM, Sarkar M et al (2017) TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95(4):808–816. https://doi.org/10.1016/j.neuron.2017.07.025. e809
Muschol M, Rosenberger F (1997) Liquid-liquid phase separation in supersaturated lysozyme solutions and associated precipitate formation/crystallization. J Chem Phys 107:1953–1962. https://doi.org/10.1063/1.474547
Sengers JV (1982) Phase transitions cargèse 1980. Universality of critical phenomena in classical fluids., vol 72. Nato Science Series B, 1 edn. Plenum, New York, NY. https://doi.org/10.1007/978-1-4613-3347-0
Stanley HE (1971) Introduction to phase transitions and critical phenomena. Oxford University Press, New York, NY
Edelhoch H (1967) Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6(7):1948–1954
Acknowledgment
This work was supported by the St. Jude Children’s Research Hospital Collaborative on Membrane-less Organelles in Health and Disease and the American Lebanese Syrian Associated Charities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Milkovic, N.M., Mittag, T. (2020). Determination of Protein Phase Diagrams by Centrifugation. In: Kragelund, B.B., Skriver, K. (eds) Intrinsically Disordered Proteins. Methods in Molecular Biology, vol 2141. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0524-0_35
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0524-0_35
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0523-3
Online ISBN: 978-1-0716-0524-0
eBook Packages: Springer Protocols