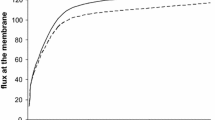
Abstract
Both skeletal and cardiac muscle cells rely heavily on the oxidation of long-chain fatty acids to utilize chemically stored energy for contractile work. Under normal conditions fatty acids are continuously supplied from the microvascular compartment to the contracting myocytes. Exogenous fatty acids are transported to muscle tissue via the blood either complexed to albumin or covalently bound in triacylglycerols forming the neutral lipid core of circulating lipoproteins such as chylomicrons or very low-density lipoproteins. The first barrier met by fatty acids on their way from the vascular compartment to the myocytes is the endothelium constituting the capillary wall. After dissociation of the albumin—fatty acid complex or release from the triacylglycerol core of lipoproteins, fatty acids most likely transverse the endothelium by crossing the luminal membrane, the cytosol, and subsequently the abluminal wall of the endothelial cell. Transfer through the interendothelial clefts or lateral diffusion within the phospholipid bilayer of the endothelial plasmalemma should be considered as inconsequential. The mechanism responsible for transmembrane movement of fatty acids is incompletely understood, although recent findings suggest the involvement of a number of membrane-associated proteins. Kinetic studies have revealed that interaction of the albumin-fatty acid complex with the endothelial membrane may accelerate the dissociation of the complex, which facilitates the uptake of fatty acids by the endothelium. Albumin-binding proteins (ABP) might be instrumental in this interaction. Moreover, plasmalemmal fatty acid-binding protein (FABPpm), fatty acid translocase (FAT) and fatty acid- transport protein (FATP) are putatively involved in transmembrane movement of the fatty acid molecules. Diffusion through the endothelial cytosol might be facilitated by a cytoplasmic fatty acid-binding protein, the type of which may be related to the epithelial fatty acid-binding protein (E-FAPBc).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Abumrad, N. A., M. R. El-Maghrabi, E.-Z. Amri, E. Lopez, and P. A. Grimaldi. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. J. Biol. Chem. 268: 17665–17668, 1993.
Antohe, F., L. Dobrila, C. Heltianu, N. Simionescu, and M. Simionescu. Albumin-binding proteins: function in the receptor-mediated binding and transcytosis of albumin across cultured endothelial cells. Eur. J. Cell Biol. 60: 268–275, 1993.
Bassingthwaighte, J. B., L. Noodleman, G. J. Van der Vusse, and J. F. C. Glatz. Modeling of palmitate transport in the heart. Mol. Cell. Biochem. 88: 51–58, 1989.
Borensztjan, J., M. S. Rone, S. P. Babirak, J. A. McGarry, and L. B. Oscai. Effect of exercise on lipoprotein lipase activity in rat heart and skeletal muscle. Am. J. Physiol. 229: 394–397, 1975.
Braun, J. E. A., and D. L. Severson. Regulation of the synthesis, processing and translocation of lipoprotein lipase. Biochem. J. 287: 337–347, 1992.
Brenner, R. R. Effect of unsaturated fatty acids on membrane structure and enzyme kinetics. Prog. Lipid Res. 23: 69–96, 1984.
Calles-Escandón, J., L. Sweet, O. Ljungqvist, and M. F. Hishman. The membrane-associated 40kD fatty acid binding protein (Berk’s protein), a putative fatty acid transporter, is present in human skeletal muscle. Life Sci. 58: 19–28, 1996.
Glatz, J. F. C., and G. J. Van der Vusse. Cellular fatty acid-binding proteins: their functions and physiological significance. Prog. Lipid Res. 35: 243–282, 1996.
Gollnick, P. D., and B. Saltin. Fuel for muscular exercise: role of fat. In: Exercise, nutrition and energy metabolism., edited by E. S. Horton and R. L. Terjung. New York: MacMillan Publ. Cy., 1988, p. 71–88.
Goresky, G. A., W. Stremmel, C. P. Rose, S. Guirguis, A. J. Schwab, H. E. Diede, and E. Ibrahim. The capillary transport system for free fatty acids in the heart. Circ. Res. 74: 1015–1026, 1994.
Greenwalt, D. W., S. H. Scheck, and T. Rhinehart-Jones. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. J. Clin. Invest. 96: 1382–1388, 1995.
Kamp, F., and J. A. Hamilton. Movement of fatty acids, fatty acid analogues, and bile acids across phos-pholipid bilayers. Biochemistry 32: 11074–11086, 1993.
Kiens, B., and H. Lithell. Lipoprotein metabolism influenced by training-induced changes in human skeletal muscle. J. Clin. Invest. 83: 558–564, 1989.
Kuikka, J., M. Levin, and J. B. Bassingthwaighte. Multiple tracer dilution estimates of D-glucose and 2-deoxy-D uptake by the heart. Am. J. Physiol. 250: H29–H42, 1986.
Linssen, M. C. J. G., W. Engels, P. J. M. R. Lemmens, V. V. T. Heynen, M. Van Bilsen, R. S. Reneman, and G. J. Van der Vusse. Production of arachidonic acid-metabolites in adult rat cardiac myocytes, endothelial cells and fibroblast-like cells. Am. J. Physiol. 264: H973–H982, 1993.
Luiken, J. J. F. P., F. A. Van Nieuwenhoven, G. America, G. J. Van der Vusse, and J. F. C. Glatz. Uptake and metabolism of palmitate by isolated cardiac myocytes from adult rats: Involvement of sarcolemmal proteins. J. Lipid Res. 38: 745–758, 1997.
Masouye, I., G. Hagens, T. H. Van Kuppeveldt, P. Madson, J.-H. Saurat, J. H. Veerkamp, M. S. Pepper, and G. Siegenthaler. Endothelial cells of the human microvasculature express epidermal fatty acid-binding proteins. Circ. Res. 81: 297–303, 1997.
Olsson, A. G., B. Eklund, L. Kaijser, and L. A. Carlson. Extraction of endogenous plasma triglycerides by the working human forearm muscle in the fasting state. Scand. J. Clin. Lab. Invest. 35: 231–236, 1975.
Richieri, G. V., A. Anel, and A. M. Kleinfeld. Interactions of long-chain fatty acid and albumin: Determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry 32: 7574–7580, 1993.
Schaffer, J. E., and H. F. Lodish. Expression, cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79: 427–436, 1994.
Schnitzer, J. E., A. Sung, R. Horvat, and J. Bravo. Preferential interaction of albumin-binding proteins, gp30and gp18, with conformationally modified albumins. Presence in many cells and tissues with a possible role in catabolism. J. Biol. Chem. 34: 24544–24553, 1992.
Scow, R. G., and E. J. Blanchette-Mackie. Why fatty acids flow in cell membranes. Prog. Lipid Res. 24: 197–241, 1985.
Spahr, B., A. Krüzfeldt, S. Mertens, B. Siegmund, and H. M. Piper. Fatty acids are not an important fuel for coronary microvascular endothelial cells. Mol. Cell. Biochem. 88: 59–64, 1989.
Spector, A. A., J. E. Fletcher, and J. D. Ashbrook. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry 10: 3229–3232, 1971.
Stremmel, W., G. Strohmeyer, F. Borchard, S. Kochwa, and P. D. Berk. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc. Natl. Acad. Sci. USA. 82: 4–8, 1985.
Terjung, R. L., and H. Kaciuba-Uscilko. Lipid metabolism during exercise: Influence of training. Diabetes/Metab. Rev. 2: 35–51, 1986.
Terjung, R. L., B. G. Mackie, G. A. Dudley, and H. Kaciuba-Uscilko. Influence of exercise on chylomicron triacylglycerol metabolism: plasma turnover and muscle uptake. Med. Sci. Sports Exerc. 15: 340–347, 1983.
Van der Vusse, G. J., J. F. C. Glatz, H. C. G. Stam, and R. S. Reneman. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol. Rev. 72: 881–940, 1992.
Van der Vusse, G. J., S. E. Little, and J. B. Bassingthwaighte. Transendothelial transport of arachidonic and palmitic acid in the isolated rabbit heart. J. Mol Cell. Cardiol. 19 (suppl.): S100 (abstract), 1987.
Van der Vusse, G. J., and R. S. Reneman. Lipid Metabolism in muscle. In: Handbook of Physiology. Integration of motor, circulatory, respiratory and metabolic control during exercise. Am. Phys. Soc., edited by L. B. Rowell and J. T. Shepherd, 1996, p. 952-994.
Van Nieuwenhoven, F. A., C. P. H. J. Verstijnen, G. J. J. M. Van Eijs, E. Van Breda, Y. F. De Jong, G. J. Van der Vusse, and J. F. C. Glatz. Fatty acid transfer across the myocardial capillary wall: No evidence for a substantial role of cytoplasmic fatty acid-binding protein. J. Mol. Cell. Cardiol. 26.: 1635–1647, 1994.
Vork, M. M., J. F. C. Glatz, and G. J. Van der Vusse. Modeling intracellular fatty acid transport: possible mechanistic ole of cytoplasmic fatty acid-binding protein. Prostagl. Leukotr. Essent. Fatty Acids 57: 11–16, 1997.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1998 Springer Science+Business Media New York
About this chapter
Cite this chapter
Van der Vusse, G.J., Glatz, J.F.C., Van Nieuwenhoven, F.A., Reneman, R.S., Bassingthwaighte, J.B. (1998). Transport of Long-Chain Fatty Acids across the Muscular Endothelium. In: Richter, E.A., Kiens, B., Galbo, H., Saltin, B. (eds) Skeletal Muscle Metabolism in Exercise and Diabetes. Advances in Experimental Medicine and Biology, vol 441. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-1928-1_17
Download citation
DOI: https://doi.org/10.1007/978-1-4899-1928-1_17
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-1930-4
Online ISBN: 978-1-4899-1928-1
eBook Packages: Springer Book Archive