Abstract
The human epithelial cell line Caco-2 has been widely used as a model of the intestinal epithelial barrier. The Caco-2 cell line is originally derived from a colon carcinoma. However, one of its most advantageous properties is its ability to spontaneously differentiate into a monolayer of cells with many properties typical of absorptive enterocytes with brush border layer as found in the small intestine. The Caco-2 cell line is heterogeneous and contains cells with slightly different properties. Thus, cultivation conditions can be expected to select for the growth of subpopulations of cells resulting in a cellular model system with properties that may differ from the original cell line. Accordingly, results obtained under similar experimental conditions in different laboratories may not be directly comparable. Due to this, a variety of cloned Caco-2 cell lines has been established, and described in the literature. This chapter will however, focus on describing how to handle and cultivate the original Caco-2 cell line as obtained from cell culture collections like American Type Culture Collection and the European Collection of Cell Cultures. Detailed protocols for handling the Caco-2 cells in the laboratory are provided. Furthermore, in Chap. 9 general protocols for measuring barrier function by transepithelial resistance (TEER), and monolayer integrity by Lucifer Yellow flux are described. Proper testing of the cell monolayer is absolutely critical in exploiting Caco-2 cells to measure interaction, uptake and cellular transport of drugs and food components.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Origin
Back in the 1970s several epithelial cell lines were established from gastrointestinal tumors. The purpose was to study mechanisms in cancer development and effects of cytotherapy. Partly due to the heterogeneity of primary intestinal epithelial cells both in morphology and function, i.e. small bowel enterocytes, goblet cells, enteroendocrine cells, Paneth cells and M-cells, there was a need for differentiating the tumor cells into more specialized cell types. Several of the cell lines could be partly differentiated by addition of synthetic or biological factors to the medium. However, one of them, Caco-2, was rather unique as it was able to differentiate spontaneously when reaching confluence. Caco-2 (Cancer coli-2) was established from a human colorectal adenocarcinoma by Jorgen Fogh at the Sloan-Kettering Cancer Research Institute (Fogh et al. 1977).
2 Features and Mechanisms
Early studies revealed that differentiated Caco-2 cells expressed several morphological and functional properties characteristic of small bowel enterocytes. Towards confluence they start to polarize acquiring a characteristic apical brush border with microvilli. Tight junctions form between adjacent cells, and they express enzyme activities typical of enterocytes, i.e. lactase, aminopeptidase N, sucrase-isomaltase and dipeptidylpeptidase IV. However, markers of colonocytes are also present in Caco-2 cells (Engle et al. 1998) (Table 10.1).
3 Stability, Consistency and Reproducibility
During the 35 years that have passed since its establishment, Caco-2 cells have been propagated in a number of laboratories worldwide. Due to different culture conditions and different numbers of passages, Caco-2 cells have often acquired different properties. Thus, the expression of differentiation markers typical of enterocytes, change with increasing numbers of passages (Artursson et al. 2001). Also, parameters like transepithelial electric resistance (TEER) and proliferation rate have been reported to increase with passage number (Briske Andersson et al. 1997). It has also been documented that late passage cells may start growing in multilayers, a phenomenon that will affect TEER measurements, and make comparisons with results based on early passage cells difficult.
4 Relevance to Human In Vivo Situation
To closer mimic the steric conditions in the intestine in vivo, Caco-2 cells are cultured on permeable filter inserts that can be obtained from a number of vendors like Becton Dickenson, Corning, Costar, etc. Cultivation of Caco-2 cells on filter supports improves their morphological and functional differentiation. It has been well documented that polarized Caco-2 monolayers represent a reliable correlate for studies on the absorption of drugs and other compounds after oral intake in humans. Several studies have compared Caco-2 permeability coefficients with absorption data in humans and found high correlation, particularly if the compounds are transported by passive paracellular transport mechanisms (Artursson and Karlsson 1991; Cheng et al. 2008; Sun et al. 2008).
Although Caco-2 cells have been found to express a large number of enzymes and transporter proteins present in normal human intestinal epithelium, recent studies suggest that there are variations between gene expression profiles of transformed epithelial cell lines like Caco-2, HT29 and normal human intestinal epithelium (Bourgine et al. 2011). Furthermore, differences are not only found between the cell lines and normal epithelium, principle component analysis of gene expression profiles reveals clear differences between the Caco-2 and HT29 cell lines, also (Bourgine et al. 2011). This is not unexpected. Normal intestinal epithelium is made up of several different cell types, and differences in gene expression profiles are not only observed in the mucosal epithelium along the whole gastrointestinal tract, but also along the crypt-villus axis (Anderle et al. 2005). Obviously, data analysis of experiments in the Caco-2 cell model cannot be directly compared with the in vivo situation. Still, intestinal epithelial cell models like the Caco-2 model, holds many advantages due to its simplicity and reproducibility allowing inter-laboratory comparison of results. Furthermore, pursuing an effect of a food bioactive in a cell line model opens for studies of molecular mechanisms which may be more difficult to address in vivo.
5 General Protocols for Caco-2 Cells
5.1 General Maintenance
Caco-2 cells are routinely maintained in RPMI 1640 (alternatively Dulbecco’s Modified Eagle Medium, DMEM) with 10 % fetal bovine serum and the following additions: 1 % non-essential amino acids (NEAA), 50 μM thioglycerol, 25 mg/ml gentamycin (complete medium). The cells should be kept at 37 °C in a humidified atmosphere containing 5 % CO2. For propagation in culture flasks, Caco-2 cells are seeded in a concentration of 105 cells/cm2. Medium should be changed every 3 days. At 80 % confluence, typically after 4–5 days, the cells are split 1:10 before further cultivation. Trypsinize Caco-2 cells by first rinsing with PBS (15 ml pr. 75 cm2 flask). Add 5 ml trypsin/EDTA solution, rinse the cells and pour off most of the trypsin/EDTA. Only 1 ml trypsin/EDTA solution is necessary to wet the whole cell layer in a 75 cm2 flask. Incubate the flask at 37 °C for 6–15 min. The incubation with trypsin should be as short as possible as this process will affect cell viability. As soon as the cells are detached, stop trypsinization by adding complete medium containing fetal calf serum. Transfer the cells to a test tube and let cell aggregates and debris sediment. Transfer the supernatant to a new test tube, and take an aliquot to count the cells. Check cell viability. The content of dead cells should not exceed 5 %.
5.2 Protocol for Polarizing Caco-2 Cells in Tissue Culture Inserts
Caco-2 cells can be cultured on a filter support (Fig. 10.1). The filter support can be made from polycarbonate, polyester or polyethylene terephthalate. Particularly the latter is claimed to be inert with low non-specific protein-binding properties. The filter supports can be transparent or translucent. If you want to follow the differentiation process in the inverted microscope and prepare the differentiated cells for scanning electron microscopy, transparent filters are preferred. Furthermore, the filters can come with different pore sizes ranging from 0.4 to 8 μm. To study parameters like transcellular transport and permeability, 0.4 μm filters are recommended. Insert with larger pore sizes can be exploited if direct cell–cell interactions are being scrutinized, i.e. in co-cultures of Caco-2 cells with stromal cells or adherent immune cells.
The figure is a drawing of the differentiation of Caco-2 cells on a tissue culture insert. After the Caco-2 cells reach confluence (middle) they start to differentiate spontaneously, and after a total culture period of around 21 days they will appear with dense microvilli on the apical side characteristic of small intestinal enterocytes
Depending on inherent properties of the particular Caco-2 cells available, it may be necessary to coat the filters with protein to prevent the cells from detaching in the final stages of the differentiation process. Some vendors offer ready-coated filters. However, efficient coating of the filters can be carried out by covering them with collagen solution Type I from Sigma (1/100 in water). Incubate at room temperature for 3–4 h. Remove the collagen solution and leave the filter inserts to dry overnight.
Place the necessary number of filter inserts in a 12 well plate. Dilute the cell suspension to a concentration of 1 × 106/ml. Seed 0.5 ml of the cell suspension to each insert corresponding to seeding density of 500,000 cells pr. 12 mm filter insert.
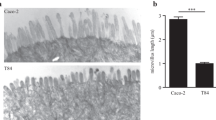
This seeding density corresponds to 4 × 105 cells/cm2. Add 1.5 ml medium to the basolateral compartment in a 12 well plate, and 0.5 ml to the apical compartment. Depending on experience it is recommended to start with setting up filters in quadruplicate, that is later reduced to triplicate or even duplicate. Medium should be changed on days 4, 8, 12, 16 and 18. Cells will be fully polarized by day 21. See SEM picture in Fig. 10.2.
The differentiation of Caco-2 cells seem to follow a time schedule in the expression of morphological and biochemical properties of the absorptive enterocytes. Due to the cellular heterogeneity of the cell line the differentiation process occurs in a mosaic pattern, with some areas expressing fully differentiated cells with microvilli after 12–14 days while other areas contain less differentiated cells. According to the protocol above, the Caco-2 monolayer will be homogenously differentiated after 18–21 days. When polarized and confluent, the cell layer forms a continuous barrier between the upper and lower compartments (apical/mucosal and basolateral/serosal). The compound of interest, suspended in a physiological fluid, typically PBS, is added to the upper compartment, and the increase in concentration in the lower compartment is measured. In this way the permeability, absorption and transepithelial transport of any nutritional compound can be studied. Special emphasis should be paid to compounds or drugs that are poorly soluble in physiological buffers. If stock solutions of the compound of interest has to be made in organic solvents like ethanol or dimethylsulfoxide (DMSO), be aware that organic solvents may affect the cell layer in various ways. Always include a filter/filters for solvent control. In general, avoid using higher solvent concentrations than 1 % (v/v) or else.
5.3 Troubleshooting Guide for Transport Experiments Across Caco-2 Monolayers
Problem | Possible explanation | Try the following |
|---|---|---|
Very high Papp | Leaky cell monolayers. | Validate integrity of monolayers (see protocol with Lucifer Yellow flux in Chap. 9). |
High expression level of transporter protein for active transport process | Protein expression levels may vary with passage number and culturing conditions. Use the same batch of cells with similar passage number for consistent results. | |
Very low Papp | Test compound degradation | Check stability of test compound under experimental conditions |
Poor solubility | Verify that the test compound is fully dissolved. | |
Membrane transporter may have become saturated | Verify the concentration of the test substance. Titrate and use lower concentration. | |
Poor mass balance | High degradation | Stability of test compound (see above) |
High cellular uptake | Analyze amount of compound in the cell monolayer by lysing the cells and extracting the compound for quantification. | |
Adsorption of compound to the filter insert or culture vessel | Monitor compound concentration before and after transfer to the filter insert system. | |
High standard deviation | Damaged cell monolayers | It is vitally important to avoid damaging the monolayers during sampling |
6 Applications
Cell culture assays has offered exciting new possibilities in many scientific disciplines. If properly used the Caco-2 cell line can provide information about the biological and biochemical basis of barrier properties of the intestinal mucosa, but may also unravel valuable information about the absorption of drugs and dietary components relevant for both the pharmaceutical and the food industry. Thus, the Caco-2 cell line has been exploited for a range of applications, among them the following:
-
To study mechanisms and effects of microbiota, microbiota metabolites, food digesta and bioactive food components on the barrier function of the intestinal epithelium (Shimizu 2010).
-
To elucidate pathways for the transport of drugs or food components (e.g. paracellular versus transcellular or passive versus carrier-mediated mechanisms) across the intestinal epithelium (Knipp et al. 1997).
-
In studying potential toxic effects of drug candidates or food metabolites in the intestinal mucosa (Chang et al. 1993).
-
To determine how components of a formulation (e.g. adjuvants or food matrices) may influence intestinal epithelial transport of bioactive molecules (Nerurkar et al. 1996).
-
In the characterization of the optimal physiochemical properties of a bioactive molecule for passive diffusion via the paracellular or transcellular pathways across the intestinal epithelium (Burton et al. 1996).
-
To study molecular details and significance of efflux systems (e.g. multi-drug resistance proteins like the P-glycoprotein) in the intestinal epithelium (Burton et al. 1997).
-
To study and determine interactions between bioactive molecules during transport across the intestinal epithelium (Wacher et al. 1996).
7 Advantages and Disadvantages
The Caco-2 cells spontaneously differentiate to express morphological (polarized columnar epithelium) and functional characteristics of mature small intestinal enterocytes. The polarized Caco-2 cell layer shows 4 times higher TEER values compared to HT29 monolayers, i.e. more similar to the in vivo situation. Caco-2 cells express most receptors, transporters and drug metabolizing enzymes like aminopeptidase, esterase and sulfatase found in normal epithelium. However, no P-450 metabolizing enzyme activity has been reported.
In comparison with normal intestinal epithelium the Caco-2 cell model have several limitations. First of all that the normal epithelium contains more than one cell type, not only enterocytes. Secondly, when using the Caco-2 cell model, no mucus and unstirred water layer is present. Furthermore, a number of non-cellular parameters will affect the absorption of a certain compound in cells. Thus, transport of lipophilic molecules is strongly influenced by the presence of bile acids and phospholipids, and also compound solubility in the mucus layer as well as the unstirred water layer close to the epithelium, will strongly influence uptake in vivo. Although Caco-2 cells in general provide a powerful tool for studying properties of the intestinal epithelium, one has to be cautious in extrapolating data from such in vitro models to the in vivo situation.
8 Conclusion
For Caco-2 cell monolayer systems to provide reliable information several critical parameters have to be controlled. First of all, it is imperative that the integrity of the monolayers is validated prior to transport studies. The protocol based on TEER measurements of Lucifer Yellow flux (Chap. 9) are crucial for confirming the quality and integrity of the polarized epithelial cell monolayer. Also, if the compound being tested is a candidate substrate for a specific transporter, the monolayer must be checked to verify the expression and activity of that particular transporter. The presence of the transporter at the mRNA level can be verified by PCR, at the protein level by immunoblotting and functionally by employing inhibitors to and known ligands to demonstrate the presence of a functional transporter. It is important to recognize that protein expression may be influenced by a variety of parameters, the most important being culture conditions, passage number or age of the cells in culture. Thus, a positive control should always be included demonstrating the presence of a functional transporter protein or protein complex. It is also important that the effect of contamination of the cell culture is eliminated. Although short time protocols for obtaining a polarized Caco-2 cell monolayer has been published, the standard and most reliable procedure requires 21 days of culture to obtain a polarized monolayer with full expression of tight junctions and other intercellular contacts. During a 3 weeks culture period and with antibiotics in the medium, a subclinical infection not identifiable even by light microscopy, may bias or obscure results completely. Thus, it is of imperative importance.Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
Anderle P, Sengstag T et al (2005) Changes in the transcriptional profile of transporters in the intestine along the anterior-posterior and crypt-villus axes. BMC Genomics 6:69–86
Artursson P, Karlsson J (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 175:880–885
Artursson P, Palm K, Luthman K (2001) Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev 46:27–43
Briske Andersson MJ, Finley JW, Newman SM (1997) The influence of culture time and passage number on the morphological and physiological development of Caco-2 cells. Proc Soc Exp Biol Med 214:248–257
Bourgine J, Billaut-Laden I et al (2012) Gene expression profiling of systems involved in the metabolism and the disposition of xenobiotics: comparison between human intestinal biopsy samples and colon cell lines. Drug Metab Dispos 40(4):694–705
Burton PS, Conradi RA et al (1996) How structural features influence the permeability of peptides. J Pharm Sci 85:1336–1340
Burton PS, Goodwin JT et al (1997) In vitro permeability of peptidomimetics: the role of polarized efflux pathways as additional barriers to absorption. Adv Drug Deliv Rev 23:143–156
Chang AS, Chikhale PJ et al (1993) Utilization of a human intestinal epithelial cell culture system (Caco-2) for evaluating cytoprotective agents. Pharm Res 10:1620–1626
Cheng K-C, Li C, Uss AS (2008) Prediction of oral drug absorption in humans from cultured cell lines and experimental animals. Expert Opin Drug Metab Toxicol 4:581–590
Engle MJ, Goetz GS, Alpers DH (1998) Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J Cell Physiol 174:362–369
Fogh J, Wright JC, Loveless JD (1977) Absence of HeLa cell contamination in 169 cell lines derived from human tumours. J Natl Cancer Inst 21:393–408
Knipp GT, Ho NFH et al (1997) Paracellular diffusion in Caco-2 monolayers: effects of perturbants on the transport of hydrophilic compounds that vary in charge and size. J Pharm Sci 86:1105–1110
Nerurkar MM, Burton PS, Borchart RT (1996) The use of surfactants to enhance the permeability of peptides through Caco-2 cells by inhibition of an apically polarized efflux system. Pharm Res 13:528–534
Shimizu M (2010) Interaction between food substances and the intestinal epithelium. Review Biosci Biotechnol Biochem 74:232–241
Sun H et al (2008) The Caco-2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol 4:395–411
Wacher VJ, Salphati L, Benet LZ (1996) Active secretion and enterocytic drug metabolism barriers to drug absorption. Adv Drug Deliv Rev 20:99–112
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Lea, T. (2015). Caco-2 Cell Line. In: Verhoeckx, K., et al. The Impact of Food Bioactives on Health. Springer, Cham. https://doi.org/10.1007/978-3-319-16104-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-16104-4_10
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15791-7
Online ISBN: 978-3-319-16104-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)