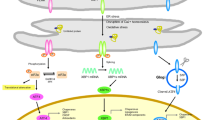
Abstract
Recent clinical, genetic and experimental evidence indicates that dysregulation of endoplasmic reticulum (ER) homeostasis plays an important role in the pathogenesis of neurodegenerative diseases and psychiatric illness. Protein flux through the ER must be carefully monitored to prevent dysregulation of ER homeostasis and stress. ER stress elicits a signaling cascade known as the unfolded protein response (UPR) which functions in influencing both cellular life and death decisions. In this chapter, we address the transition from the physiological ER stress response to the pathological response and explore the mechanisms of ER stress-mediated dysfunction and death of neurons during the progression of neurodegenerative diseases and psychiatric illness.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ER:
-
Endoplasmic reticulum
- UPR:
-
Unfolded protein response
- PERK:
-
PKR-like ER kinase
- CHOP:
-
C/EBP Thomologous protein
- eIF2a:
-
Eukaryotic initiation factor 2a
- PD:
-
Parkinson’s disease
- AJ-PD:
-
Autosomal recessive juvenile-onset Parkinson’s disease
- Pael-R:
-
Pael-receptor
- SPG:
-
Spastic paraplegia
- BSCL2:
-
Berardinelli-Seip congenital lipodystrophy 2
- ALS:
-
Amyotrophic lateral sclerosis
- SOD1:
-
Superoxide dismutase 1
- PDI:
-
Protein disulfide isomerase
- PUMA:
-
P53-upregulated mediator of apoptosis
- RP:
-
Retinitis pigmentosa
- ERAD:
-
ER associated degradation
- AD:
-
Alzheimer’s disease
- PS:
-
Presenilin
- IRE1:
-
Inositol-requiring enzyme 1
- NMDA:
-
N-methy D-aspartate
- NOS:
-
nitric oxide synthase
- XBP1:
-
X-box binding protein 1
- CHOP:
-
CCAAT/enhancer binding protein homologous protein
- MSS:
-
Marinesco-Sjogren syndrome
- IBMPFD:
-
Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia
- αSyn:
-
α-synuclein
- SERCA:
-
Sarco-endoplasmic reticulum Ca2 + ATPase pump
- IP3 receptor:
-
Inositol triphosphate-gated channel
- SCA:
-
Spinocerebeller atrophy
- OASIS:
-
Old astrocyte specifically-induced substrate
- ATF6:
-
Activating transcription factor 6
- CLN1:
-
Neuronal ceroid lipofuscinosis 1
- PPT1:
-
Palmitoyl protein thioesterase 1
- TMAO:
-
Trimethylamine N-oxide
- TUDCA:
-
Tauroursodeoxycholic acid
- PMD:
-
Pelizaeus Merzbacher disease
- PLP1:
-
Proteolipid protein-1
- P0:
-
Myelin protein zero
- VWMD:
-
Vanishing White Matter Disease
- GEF:
-
Guanine nucleotide exchanging factor
- ASD:
-
Autism spectrum disorder
- CADM1:
-
Cell adhesion molecule-1
- DIDMOAD:
-
Diabetes Insipidus, Diabetes Mellitus, Optic Atrophy and Deafness
- WFS1:
-
Wolfram syndrome 1
- CISD2:
-
CDGSH iron sulfur domain 2
- MRI:
-
Magnetic resonance imaging
- 4PBA:
-
4-phenylbutylate
References
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10:10–7
Tong J, Wong H, Guttman M et al (2010) Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: a comparative investigation. Brain 133:172–88
Bennett EJ, Shaler TA, Woodman B et al (2007) Global changes to the ubiquitin system in Huntington’s disease. Nature 448:704–708
O’Brien L, Shelley K, Towfighi J et al (1980) Crystalline ribosomes are present in brains from senile humans. Proc Natl Acad Sci USA 77:2260–2264
Douglas PM, Dillin A (2010) Protein homeostasis and aging in neurodegenration. J Cell Biol 190:719–729
Zabel C, Nguyen HP, Hin SC et al (2010) Proteasome and oxidative phosphorylation changes may explain why aging is a risk factor for neurodegenerative disorders. J Proteomics 73:2230–2238
Naidoo N, Ferber M, Master M et al (2008) Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci 28:6539–65348
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363: 1783–1793
Reichmann H (2010) Clinical criteria for the diagnosis of Parkinson’s disease. Neurodegener Dis 7:284–290
Kitada T, Asakawa S, Hattori N et al (1988) Mutations in parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608
Imai Y, Soda M, Inoue H et al (2000) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105:891–902
Murakami T, Shoji M, Imai Y et al (2004) Pael-R is accumulated in Lewy bodies of Parkinson’s disease. Ann Neurol 55: 439–442
Fei W, Du X, Yang H (2011) Seipin, adipogenesis and lipid droplets. Trends Endocrinol Metab 22:204–210
Windpassinger C, Auer-Grumbach M, Irobi J et al (2004) Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet 36:271–276
Braakman I, Bulleid NJ (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 80:71–99
Ito D, Suzuki N (2007) Molecular pathogenesis of seipin/BSCL-2-related motor neuron diseases. Ann Neurol 61: 237–250
Ito D, Fujisawa T, Iida H et al (2008) Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17. Neurobiol Dis 31:266–277
Kiernan MC, Vucic S, Cheah BC et al (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955
Rosen DR, Siddique T, Patterson D et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59–62
Buettner GR (2011) Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem 11:341–346
ALSOD ALS online genetics database. http://alsod.iop.kcl.ac.uk/. Accessed 26 April 2012
Shefner JM, Reaume AG, Flood DG et al (1999) Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology 53:1239–1246
Gurney ME, Pu H, Chiu AY et al (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264:1772–1175
Kikuchi H, Almer G, Yamashita S et al (2006) Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci USA 103:6025–6030
Atkin JD, Farg MA, Turner BJ et al (2006) Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J Biol Chem 281:30152–30165
Kieran D, Woods I, Villunger A et al (2007) Deletion of the BH3-only protein puma protects motoneurons from ER stress-induced apoptosis and delays motoneuron loss in ALS mice. Proc Natl Acad Sci USA 104:20606–20611
Reimertz C, Kögel D, Rami A et al (2003) Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol 162:587–597
Saxena S, Cabuy E, Caroni P (2009) A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci 12:627–636
Sahel J, Bonnel S, Mrejen S et al (2010) Retinitis pigmentosa and other dystrophies. Dev Ophthalmol 47:160–167
Malanson KM, Lem J (2009) Rhodopsin-mediated retinitis pigmentosa. Prog Mol Biol Transl Sci 88:1–31
Lin JH, Li H, Yasumura D et al (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318:944–949
Gorbatyuk MS, Knox T, LaVail MM et al (2010) Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci USA 107:5961–5966
Kang MJ, Ryoo HD (2009) Suppression of retinal degeneration in Drosophila by stimulation of ER-associated degradation. Proc Natl Acad Sci USA 106:17043–17048
Villemagne VL, Pike KE, Chételat G et al (2011) Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol 69:181–92
Ballard C, Gauthier S, Corbett A et al (2011) Alzheimer’s disease. Lancet 377:1019–1031
Katayama T, Imaizumi K, Sato N et al (1999) Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol 1:479–485
Terro F, Czech C, Esclaire F (2002) Neurons overexpressing mutant presenilin-1 are more sensitive to apoptosis induced by endoplasmic reticulum-Golgi stress. J Neurosci Res 69:530–539
Sato N, Urano F, Yoon Leem J et al (2000) Upregulation of BiP and CHOP by the unfolded-protein response is independent of presenilin expression. Nat Cell Biol 2:863–870
Margittai E, Bánhegyi G (2010) Oxidative folding in the endoplasmic reticulum: towards a multiple oxidant hypothesis? FEBS Lett 584:2995–1998
Honjo Y, Kaneko S, Ito H et al (2011) Protein disulfide isomerase-immunopositive inclusions in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12:444–450
Wilhelmus MM, Verhaar R, Andringa G et al (2011) Presence of tissue transglutaminase in granular endoplasmic reticulum is characteristic of melanized neurons in Parkinson’s disease brain. Brain Pathol 21:130–139
Uehara T, Nakamura T, Yao D et al (2006) S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441:513–517
Gething MJ (1999) Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol 10:465–472
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
Alder NN, Shen Y, Brodsky JL et al (2005) The molecular mechanisms underlying BiP-mediated gating of the Sec61 translocon of the endoplasmic reticulum. J Cell Biol 168:389–399
Yan M, Li J, Sha B (2011) Structural analysis of the Sil1-Bip complex reveals the mechanism for Sil1 to function as a nucleotide-exchange factor. Biochem J 438:447–455
Senderek J, Krieger M, Stendel C et al (2005) Mutations in SIL1 cause Marinesco-Sjögren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet 37:1312–1314
Anttonen AK, Mahjneh I, Hämäläinen RH et al (2005) The gene disrupted in Marinesco-Sjögren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet 37:1309–1311
Zhao L, Longo-Guess C, Harris BS et al (2005) Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet 37:974–979
Bernasconi R, Molinari M (2011) ERAD and ERAD tuning: disposal of cargo and of ERAD regulators from the mammalian ER. Curr Opin Cell Biol 23:176–183
Oda Y, Okada T, Yoshida H et al (2006) Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J Cell Biol 172:383–393
Nishitoh H, Kadowaki H, Nagai A et al (2008) ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev 22:1451–1464
Mori A, Yamashita S, Uchino K et al (2011) Derlin-1 overexpression ameliorates mutant SOD1-induced endoplasmic reticulum stress by reducing mutant SOD1 accumulation. Neurochem Int 58:344–353
Wang Q, Song C, Li CC (2004) Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol 146:44–57
Wójcik C, Rowicka M, Kudlicki A et al (2006) Valosin-containing protein (p97) is a regulator of endoplasmic reticulum stress and of the degradation of N-end rule and ubiquitin-fusion degradation pathway substrates in mammalian cells. Mol Biol Cell 17:4606–4618
Watts GD, Wymer J, Kovach MJ et al (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36:377–381
Johnson JO, Mandrioli J, Benatar M et al (2010) Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68:857–864
Weihl CC, Dalal S, Pestronk A et al (2006) Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum Mol Genet 15:189–199
Cummings CJ, Zoghbi HY (2000) Trinucleotide repeats: mechanisms and pathophysiology. Annu Rev Genomics Hum Genet 1:281–328
Nishitoh H, Matsuzawa A, Tobiume K et al (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16:1345–1355
Price BD, Mannheim-Rodman LA, Calderwood SK (1992) Brefeldin A, thapsigargin, and AIF4- stimulate the accumulation of GRP78 mRNA in a cycloheximide dependent manner, whilst induction by hypoxia is independent of protein synthesis. J Cell Physiol 152:545–552
Cooper AA, Gitler AD, Cashikar A et al (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313:324–328
Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047
Ono K, Ikeda T, Takasaki J et al (2011) Familial Parkinson disease mutations influence α-synuclein assembly. Neurobiol Dis 43:715–724
Ahn TB, Kim SY, Kim JY et al (2008) alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology 70:43–49
Gleichmann M, Mattson MP (2011) Neuronal calcium homeostasis and dysregulation. Antioxid Redox Signal 14:1261–1273
Michalak M, Robert Parker JM, Opas M (2002) Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32:269–278
Li WW, Alexandre S, Cao X et al (1993) Transactivation of the grp78 promoter by Ca2+ depletion. A comparative analysis with A23187 and the endoplasmic reticulum Ca(2+)-ATPase inhibitor thapsigargin. J Biol Chem 268:12003–12009
Mikoshiba K (2011) Role of IP3 receptor in development. Cell Calcium 49:331–340
Matsumoto M, Nakagawa T, Inoue T et al (1996) Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature 379:168–171
van de Leemput J, Chandran J, Knight MA et al (2007) Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet 3:e108
Hara K, Shiga A, Nozaki H et al (2008) Total deletion and a missense mutation of ITPR1 in Japanese SCA15 families. Neurology 71:547–551
Higo T, Hamada K, Hisatsune C et al (2010) Mechanism of ER stress-induced brain damage by IP(3) receptor. Neuron 68:865–878
Cheung KH, Shineman D, Müller M et al (2008) Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58:871–883
Cheung KH, Mei L, Mak DO et al (2010) Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal 3:ra22
Nave KA, Trapp BD (2008) Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci 31: 535–561
Pechan PA, Chowdhury K, Seifert W (1992) Free radicals induce gene expression of NGF and bFGF in rat astrocyte culture. Neuroreport 3:469–472
Kondo S, Murakami T, Tatsumi K et al (2005) OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol 7:186–194
Murakami T, Kondo S, Ogata M et al (2006) Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem 96:1090–1100
Jalanko A, Braulke T(2009) Neuronal ceroid lipofuscinoes. Biochim Biophys Acta 1793:697–709
Mitchison HM, Hofmann SL, Becerra CH et al (1998) Mutations in the palmitoyl-protein thioesterase gene (PPT; CLN1) causing juvenile neuronal ceroid lipofuscinosis with granular osmiophilic deposits. Hum Mol Genet 7:291–297
Zhang Z, Lee YC, Kim SJ et al (2006) Palmitoyl-protein thioesterase-1 deficiency mediates the activation of the unfolded protein response and neuronal apoptosis in INCL. Hum Mol Genet 15:337–346
Wei H, Kim SJ, Zhang Z et al (2008) ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet 17:469–477
Woodward KJ (2008) The molecular and cellular defects underlying Pelizaeus-Merzbacher disease. Expert Rev Mol Med 10:e14
Dhaunchak AS, Nave KA (2007) A common mechanism of PLP/DM20 misfolding causes cysteine-mediated endoplasmic reticulum retention in oligodendrocytes and Pelizaeus-Merzbacher disease. Proc Natl Acad Sci U S A 104:17813–17818
Gow A, Southwood CM, Lazzarini RA (1998) Disrupted proteolipid protein trafficking results in oligodendrocyte apoptosis in an animal model of Pelizaeus-Merzbacher disease. J Cell Biol 140:925–934
Southwood CM, Garbern J, Jiang W et al (2002) The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron 36:585–596
Hübner CA, Orth U, Senning A et al (2005) Seventeen novel PLP1 mutations in patients with Pelizaeus-Merzbacher disease. Hum Mutat 25:321–322
Roboti P, Swanton E, High S (2009) Differences in endoplasmic-reticulum quality control determine the cellular response to disease-associated mutants of proteolipid protein. J Cell Sci 122:3942–3953
Berger P, Niemann A, Suter U (2006) Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease). Glia 54:243–257
Giese KP, Martini R, Lemke G et al (1992) Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell 71:565–576
Xu W, Manichella D, Jiang H et al (2000) Absence of P0 leads to the dysregulation of myelin gene expression and myelin morphogenesis. J Neurosci Res 60(6):714–724
Kulkens T, Bolhuis PA, Wolterman RA et al (1993) Deletion of the serine 34 codon from the major peripheral myelin protein P0 gene in Charcot-Marie-Tooth disease type 1B. Nat Gnet 5:35–39
Pennuto M, Tinelli E, Malaguti M et al (2008) Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron 57:393–405
Asano K, Clayton J, Shalev A et al (2000) A multifactor complex of eukaryotic initiation factors, eIF1, eIF2, eIF3, eIF5, and initiator tRNA(Met) is an important translation initiation intermediate in vivo. Genes Dev 14:2534–2546
Mohammad-Qureshi SS, Jennings MD et al (2008) Clues to the mechanism of action of eIF2B, the guanine-nucleotide-exchange factor for translation initiation. Biochem Soc Trans 36:658–664
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Sheikh MS, Fornace AJ Jr (1999) Regulation of translation initiation following stress. Oncogene 18:6121–6128
Van Der Knaap MS, Pronk JC et al (2006) Vanishing white matter disease. Lancet Neurol 5:413–423
Kantor L, Pinchasi D, Mintz M et al (2008) A point mutation in translation initiation factor 2B leads to a continuous hyper stress state in oligodendroglial-derived cells. PLoS One 3:e3783
Van Der Voorn JP, van Kollenburg B, Bertrand G et al (2005) The unfolded protein response in vanishing white matter disease. J Neuropathol Exp Neurol 64:770–775
Li W, Wang X, Van Der Knaap MS et al (2004) Mutations linked to leukoencephalopathy with vanishing white matter impair the function of the eukaryotic initiation factor 2B complex in diverse ways. Mol Cell Biol 24:3295–3306
WHO International Consortium in Psychiatric Epidemiology (2000) Cross-national comparisons of the prevalences and correlates of mental disorders. Bull World Health Organ 78:413–426
Al-Qabandi M, Gorter JW, Rosenbaum P (2011) Early autism detection: are we ready for routine screening? Pediatrics 128:e211–217
Newschaffer CJ, Croen LA, Daniels J et al (2007) The Epidemiology of Autism Spectrum Disorders. Annu Rev Public Health 28:235–258
Zhiling Y, Fujita E, Tanabe Y et al (2008) Mutations in the gene encoding CADM1 are associated with Autism spectrum disorder. Biochem Biophys Res Commun 377:926–929
Fujita E, Dai H, Tanabe Y et al (2010) Autism spectrum disorder is related to endoplasmic reticulum stress induced by mutations in the synaptic cell adhesion molecule, CADM1. Cell Death Dis 1:e47
Momoi T, Fujita E, Senoo H et al (2009) Genetic factors and epigenetic factors for autism: endoplasmic reticulum stress and impaired synaptic function. Cell Biol Int 34:13–19
Frye MA (2011) Bipolar disorder—A Focus on Depression. N Engl J Med 364:51–59
Yoshida H, Matsui T, Yamamoto A et al (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891
Kakiuchi C, Iwamoto K, Ishiwata M et al (2003) Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat Genet 35:171–175
Cichon S, Buervenich S, Kirov G et al (2004) Lack of support for a genetic association of the XBP1 promoter polymorphism with bipolar disorder in probands of European origin. Nat Genet 36:783–784
Kakiuchi C, Ishiwata M, Nanko S et al (2005) Functional polymorphisms of HSPA5: possible association with bipolar disorder. Biochem Biophys Res Commun 336:1136–1143
Wolfram DJ, Wagener HP (1938) Diabetes mellitus and simple optic atrophy among siblings: report of four cases. Mayo Clin Proc 13:715–718
Barrett TG, Bundey SE, Macleod AF (1995) Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet 346:1458–1463
Fraser FC, Gunn T (1977) Diabetes mellitus, diabetes insipidus, and optic atrophy. An autosomal recessive syndrome? J Med Genet 14:190–193
Inoue H, Tanizawa Y, Wasson J et al (1998) A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 20:143–148
Amr S, Heisey C, Zhang M et al (2007) A homozygous mutation in a novel zinc-finger protein, ERIS, is responsible for Wolfram syndrome 2. Am J Hum Genet 81:673–683
Fonseca SG, Fukuma M, Lipson KL et al (2005) WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem 280:39609–39615
Fonseca SG, Ishigaki S, Oslowski CM et al (2010) Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest 120:744–755
Swift RG, Sadler DB, Swift M (1990) Psychiatric findings in Wolfram syndrome homozygotes. Lancet 336: 667–669
Swift M, Swift RG (2005) Wolframin mutations and hospitalization for psychiatric illness. Mol Psychiatry 10:799–803
Chaussenot A, Bannwarth S, Rouzier C et al (2011) Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann Neurol 69:501–508
Boyce M, Bryant KF, Jousse C (et al) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307:935–939.
Oh YK, Shin KS, Yuan J et al (2008) Superoxide dismutase 1 mutants related to amyotrophic lateral sclerosis induce endoplasmic stress in neuro2a cells. J Neurochem 104:993–1005
Reijonen S, Putkonen N, Nørremølle A et al (2008) Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp Cell Res 314:950–960
Zhu Y, Fenik P, Zhan G et al (2008) Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J Neurosci 28:2168–78
Ozcan U, Yilmaz E, Ozcan L (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140
Keene CD, Rodrigues CM Eich T et al (2002) Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci U S A 99:10671–10676
Duan WM, Rodrigues CM, Zhao LR et al (2002) Tauroursodeoxycholic acid improves the survival and function of nigral transplants in a rat model of Parkinson’s disease. Cell Transplant 11:195–205
Fernández-Sánchez L, Lax P, Pinilla I et al (2011) Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest Ophthalmol Vis Sci 52:4998–5008
Kubota K, Niinuma Y, Kaneko M et al (2006) Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem 97:1259–1268
Chuang DM (2005) The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci 1053:195–204
Wang JF, Bown C, Young LT (1999) Differential display PCR reveals novel targets for the mood-stabilizing drug valproate including the molecular chaperone GRP78. Mol Pharmacol 55:521–527
Hiroi T, Wei H, Hough C et al (2005) Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J 5:102–111
Acknowledgements
Work in the laboratory of F. Urano is supported by grants from NIH-NIDDK (R01DK067493), the Diabetes and Endocrinology Research Center at the University of Massachusetts Medical School (5 P30 DK32520), and the Juvenile Diabetes Research Foundation International (1-2008-593 and 40-2011-14). K.K. is supported by Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Kanekura, K., Lu, S., Lipson, K., Urano, F. (2012). Role of ER Stress in Dysfunction of the Nervous System. In: Agostinis, P., Afshin, S. (eds) Endoplasmic Reticulum Stress in Health and Disease. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4351-9_13
Download citation
DOI: https://doi.org/10.1007/978-94-007-4351-9_13
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4350-2
Online ISBN: 978-94-007-4351-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)