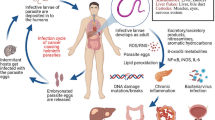
Abstract
Parasitic infection remains as a persistent public health problem and can be carcinogenic. Three helminth parasites, namely, Clonorchis sinensis (liver fluke) and Opisthorchis viverrini as well as Schistosoma haematobium (blood fluke), are classified as Group 1 carcinogens by the World Health Organization’s International Agency for Research on Cancer (IARC Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis), World Health Organization, International Agency for Research on Cancer, 2011). Infection by these parasites is frequently asymptomatic and is thus rarely diagnosed at early exposure. Persistent infection can cause severe cancer complications. Until now, the cellular and molecular mechanisms linking fluke infections to cancer formation have yet to be defined, although many studies have focused on these mechanisms in recent years, and numerous findings were made in various aspects of parasite-associated cancers. Herein, we only introduce the fluke-induced cholangiocarcinoma (CCA) and bladder carcinoma and mainly focus on key findings in the last 5 years.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
IARC. (2011) Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis), World Health Organization, International Agency for Research on Cancer
Petney TN, Andrews RH, Saijuntha W, Wenz-Mucke A, Sithithaworn P (2013) The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol 43(12–13):1031–1046
Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P et al (2012) The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int 61(1):10–16
Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A et al (2011) Opisthorchiasis and opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop 120(Suppl 1):S158–S168
Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C (2014) Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci 21(5):301–308
Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E et al (2012) The tumorigenic liver fluke Opisthorchis viverrini – multiple pathways to cancer. Trends Parasitol 28(10):395–407
Fedorova OS, Kovshirina YV, Kovshirina AE, Fedotova MM, Deev IA, Petrovskiy FI, et al. Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: a review of medical statistics. Parasitol Int. 2016;S1383–5769(16):30236–30237
Wilcox BA, Echaubard P. Balancing biomedical and ecological perspectives in research framing of liver fluke and cholangiocarcinoma in NE Thailand. Parasitol Int. 2016;S1383–5769(16):30258–30256
Xia J, Jiang SC, Peng HJ (2015) Association between liver fluke infection and hepatobiliary pathological changes: a systematic review and meta-analysis. PLoS One 10(7):e0132673
Intajarurnsan S, Khuntikeo N, Chamadol N, Thinkhamrop B, Promthet S (2016) Factors associated with periductal fibrosis diagnosed by ultrasonography screening among a high risk population for cholangiocarcinoma in Northeast Thailand. Asian Pac J Cancer Prev 17(8):4131–4136
Silakit R, Loilome W, Yongvanit P, Thongchot S, Sithithaworn P, Boonmars T, et al. Urinary microRNA-192 and microRNA-21 as potential indicators for liver fluke-associated cholangiocarcinoma risk group. Parasitol Int. 2015;S1383–5769(15):00165–00168
Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W et al (2012) Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet 44(6):690–693
Yothaisong S, Thanee M, Namwat N, Yongvanit P, Boonmars T, Puapairoj A et al (2014) Opisthorchis Viverrini infection activates the PI3K/ AKT/PTEN and Wnt/β-catenin signaling pathways in a Cholangiocarcinogenesis model. Asian Pac J Cancer Prev 15(23):10463–10468
Sriraksa R, Zeller C, El-Bahrawy MA, Dai W, Daduang J, Jearanaikoon P et al (2011) CpG-island methylation study of liver fluke-related cholangiocarcinoma. Br J Cancer 104(8):1313–1318
Chaiyadet S, Smout M, Johnson M, Whitchurch C, Turnbull L, Kaewkes S et al (2015) Excretory/secretory products of the carcinogenic liver fluke are endocytosed by human cholangiocytes and drive cell proliferation and IL6 production. Int J Parasitol 45(12):773–781
Botelho MC, Alves H, Richter J (2016) Wound healing and cancer progression in Opisthorchis viverrini associated cholangiocarcinoma. Parasitol Res 115(7):2913–2914
Chaiyadet S, Sotillo J, Smout M, Cantacessi C, Jones MK, Johnson MS et al (2015) Carcinogenic liver fluke secretes extracellular vesicles that promote cholangiocytes to adopt a tumorigenic phenotype. J Infect Dis 212(10):1636–1645
Matchimakul P, Rinaldi G, Suttiprapa S, Mann VH, Popratiloff A, Laha T et al (2015) Apoptosis of cholangiocytes modulated by thioredoxin of carcinogenic liver fluke. Int J Biochem Cell Biol 65:72–80
Surapaitoon A, Suttiprapa S, Khuntikeo N, Pairojkul C, Sripa B (2017) Cytokine profiles in Opisthorchis viverrini stimulated peripheral blood mononuclear cells from cholangiocarcinoma patients. Parasitol Int 66(1):889–892
Thanee M, Loilome W, Techasen A, Sugihara E, Okazaki S, Abe S et al (2016) CD44 variant-dependent redox status regulation in liver fluke-associated cholangiocarcinoma: a target for cholangiocarcinoma treatment. Cancer Sci 107(7):991–1000
Chng KR, Chan SH, Ng AH, Li C, Jusakul A, Bertrand D et al (2016) Tissue microbiome profiling identifies an enrichment of specific enteric bacteria in Opisthorchis Viverrini associated cholangiocarcinoma. EBioMedicine 8:195–202
Botelho MC, Vale N, Gouveia MJ, Rinaldi G, Santos J, Santos LL et al (2013) Tumour-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: an oestrogen-DNA adducts mediated pathway? Int J Parasitol 43(1):17–26
van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD et al (2003) Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop 86(2–3):125–139
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118(12):3030–3044
Hodder SL, Mahmoud AA, Sorenson K, Weinert DM, Stein RL, Ouma JH et al (2000) Predisposition to urinary tract epithelial metaplasia in Schistosoma haematobium infection. Am J Trop Med Hyg 63(3–4):133–138
Stavitsky AB (2004) Regulation of granulomatous inflammation in experimental models of schistosomiasis. Infect Immun 72(1):1–12
Pearce EJ, MacDonald AS (2002) The immunobiology of schistosomiasis. Nat Rev Immunol 2(7):499–511
Mostafa MH, Sheweita SA, O'Connor PJ (1999) Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev 12(1):97–111
Zheng YL, Amr S, Saleh DA, Dash C, Ezzat S, Mikhail NN et al (2012) Urinary bladder cancer risk factors in Egypt: a multicenter case-control study. Cancer Epidemiol Biomark Prev 21(3):537–546
Salem S, Mitchell RE, El-Alim El-Dorey A, Smith JA, Barocas DA (2011) Successful control of schistosomiasis and the changing epidemiology of bladder cancer in Egypt. BJU Int 107(2):206–211
Shiff C, Veltri R, Naples J, Quartey J, Otchere J, Anyan W et al (2006) Ultrasound verification of bladder damage is associated with known biomarkers of bladder cancer in adults chronically infected with Schistosoma haematobium in Ghana. Trans R Soc Trop Med Hyg 100(9):847–854
Gouveia MJ, Santos J, Brindley PJ, Rinaldi G, Lopes C, Santos LL et al (2015) Estrogen-like metabolites and DNA-adducts in urogenital schistosomiasis-associated bladder cancer. Cancer Lett 359(2):226–232
Santos J, Fernandes E, Ferreira JA, Lima L, Tavares A, Peixoto A et al (2014) P53 and cancer-associated sialylated glycans are surrogate markers of cancerization of the bladder associated with Schistosoma haematobium infection. PLoS Negl Trop Dis 8(12):e3329
Botelho MC, Alves H, Barros A, Rinaldi G, Brindley PJ, Sousa M (2015) The role of estrogens and estrogen receptor signaling pathways in cancer and infertility: the case of schistosomes. Trends Parasitol 31(6):246–250
Nair SS, Bommana A, Bethony JM, Lyon AJ, Ohshiro K, Pakala SB et al (2011) The metastasis-associated protein-1 gene encodes a host permissive factor for schistosomiasis, a leading global cause of inflammation and cancer. Hepatology 54(1):285–295
Botelho MC, Soares R, Vale N, Ribeiro R, Camilo V, Almeida R et al (2010) Schistosoma haematobium: identification of new estrogenic molecules with estradiol antagonistic activity and ability to inactivate estrogen receptor in mammalian cells. Exp Parasitol 126(4):526–535
Botelho MC, Ribeiro R, Vale N, Oliveira P, Medeiros R, Lopes C et al (2012) Inactivation of estrogen receptor by Schistosoma haematobium total antigen in bladder urothelial cells. Oncol Rep 27(2):356–362
Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S et al (1997) Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci U S A 94(20):10937–10942
Cavalieri EL, Rogan EG (2011) Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J Steroid Biochem Mol Biol 125(3–5):169–180
Ewald PW (2009) An evolutionary perspective on parasitism as a cause of cancer. Adv Parasitol 68:21–43
Vittecoq M, Elguero E, Lafferty KD, Roche B, Brodeur J, Gauthier-Clerc M et al (2012) Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect Genet Evol 12(2):496–498
Thirugnanam S, Rout N, Gnanasekar M (2013) Possible role of Toxoplasma gondii in brain cancer through modulation of host microRNAs. Infect Agent Cancer 8(1):8
Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, Missé D (2012) Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol Lett 8(1):101–103
Dirkx M, Boyer MP, Pradhan P, Brittingham A, Wilson WA (2014) Expression and characterization of a β-fructofuranosidase from the parasitic protist Trichomonas vaginalis. BMC Biochem 15:12
Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC et al (2013) Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 40(3):187–193
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Shui IM, Kolb S, Hanson C, Sutcliffe S, Rider JR, Stanford JL (2016) Trichomonas vaginalis infection and risk of advanced prostate cancer. Prostate 76(7):620–623
Zhu Z, Davidson KT, Brittingham A, Wakefield MR, Bai Q, Xiao H et al (2016) Trichomonas vaginalis: a possible foe to prostate cancer. Med Oncol 33(10):115
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Feng, M., Cheng, X. (2017). Parasite-Associated Cancers (Blood Flukes/Liver Flukes). In: Cai, Q., Yuan, Z., Lan, K. (eds) Infectious Agents Associated Cancers: Epidemiology and Molecular Biology. Advances in Experimental Medicine and Biology, vol 1018. Springer, Singapore. https://doi.org/10.1007/978-981-10-5765-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-5765-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5764-9
Online ISBN: 978-981-10-5765-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)