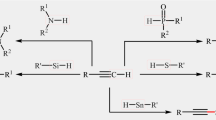
Abstract
Due to the richness of the field of cross-dehydrogenative coupling reactions and the plethora of applications toward the synthesis and derivatization of heterocyclic molecules, there is an increasing amount of synthetic methodologies which spread across different catalytic fields and hence, yield to a huge variety of operating mechanisms. Although all the pathways follow the same underlying principles, they can be easily differentiated according to the type of activation (thermal of photocatalyzed) and the type of catalyst (metallic or metal-free). In this account, we have summarized the most recent and relevant examples under the aforementioned categories, focusing especially on those presenting the most compelling and better understood mechanisms.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ackermann L (2014) Carboxylate-assisted ruthenium-catalyzed alkyne annulations by C–H/Het-H bond functionalizations. Acc Chem Res 47:281–295. https://doi.org/10.1021/ar3002798
Ackermann L, Lygin AV, Hofmann N (2011) Ruthenium-catalyzed oxidative synthesis of 2-pyridones through C–H/N–H bond functionalizations. Org Lett 13:3278–3281. https://doi.org/10.1021/ol201244s
Ackermann L, Pospech J (2011) Ruthenium-catalyzed oxidative C–H bond alkenylations in water: expedient synthesis of annulated lactones. Org Lett 13:4153–4155. https://doi.org/10.1021/ol201563r
Aggarwal T, Kumar S, Verma AK (2016) Iodine-mediated synthesis of heterocycles via electrophilic cyclization of alkynes. Org Biomol Chem 14:7639–7653. https://doi.org/10.1039/c6ob01054g
Almasalma AA, Mejia E (2018) Copper-catalyzed allylic C–H alkynylation by cross-dehydrogenative coupling. Chem Eur J 24:12269–12273. https://doi.org/10.1002/chem.201801772
Armaroli N (2001) Photoactive mono- and polynuclear Cu(i)–phenanthrolines. A viable alternative to Ru(ii)–polypyridines? Chem Soc Rev 30:113–124. https://doi.org/10.1039/b000703j
Barnard JR, Jackman LM (1960) 622. Hydrogen transfer. Part X. The dehydrogenation of hydroaromatic hydrocarbons by quinones: theoretical calculations for possible intermediates. J Chem Soc 3110. https://doi.org/10.1039/jr9600003110
Baslé O, Li C-J (2007) Copper catalyzed oxidative alkylation of sp3 C–H bond adjacent to a nitrogen atom using molecular oxygen in water. Green Chem 9:1047. https://doi.org/10.1039/b707745a
Bi HP, Chen WW, Liang YM, Li CJ (2009) A novel iron-catalyzed decarboxylative Csp3–Csp2 coupling of proline derivatives and naphthol. Org Lett 11:3246–3249. https://doi.org/10.1021/ol901129v
Boess E, Sureshkumar D, Sud A, Wirtz C, Fares C, Klussmann M (2011) Mechanistic studies on a Cu-catalyzed aerobic oxidative coupling reaction with N-phenyl tetrahydroisoquinoline: structure of intermediates and the role of methanol as a solvent. J Am Chem Soc 133:8106–8109. https://doi.org/10.1021/ja201610c
Borpatra PJ, Deb ML, Baruah PK (2018) Copper-catalyzed tandem multi-component approach to 1,3-oxazines at room temperature by cross-dehydrogenative coupling using methanol as C1 feedstock. Synlett 29:1171–1175. https://doi.org/10.1055/s-0036-1591775
Buldt LA, Guo X, Prescimone A, Wenger OS (2016) A molybdenum(0) isocyanide analogue of Ru(2,2′-Bipyridine)3 (2+): a strong reductant for photoredox catalysis. Angew Chem Int Ed Engl 55:11247–11250. https://doi.org/10.1002/anie.201605571
Buldt LA, Wenger OS (2017) Chromium complexes for luminescence, solar cells, photoredox catalysis, upconversion, and phototriggered NO release. Chem Sci 8:7359–7367. https://doi.org/10.1039/c7sc03372a
Campos KR (2007) Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem Soc Rev 36:1069–1084. https://doi.org/10.1039/b607547a
Carrick WL, Karapinka GL, Kwiatkowski GT (1969) Oxidative coupling of phenols using vanadium tetrachloride and vanadium oxytrichloride. J Org Chem 34:2388–2392. https://doi.org/10.1021/jo01260a029
Chang HR, McCusker JK, Toftlund H, Wilson SR, Trautwein AX, Winkler H, Hendrickson DN (1990) [Tetrakis(2-pyridylmethyl)ethylenediamine]iron(II) perchlorate, the first rapidly interconverting ferrous spin-crossover complex. J Am Chem Soc 112:6814–6827. https://doi.org/10.1021/ja00175a012
Chen B, Wu L-Z, Tung C-H (2018) Photocatalytic activation of less reactive bonds and their functionalization via hydrogen-evolution cross-couplings. Acc Chem Res. https://doi.org/10.1021/acs.accounts.8b00267
Chen W, Xie Z, Zheng H, Lou H, Liu L (2014) Structurally diverse alpha-substituted benzopyran synthesis through a practical metal-free C(sp3)–H functionalization. Org Lett 16:5988–5991. https://doi.org/10.1021/ol503004a
Chen WL, Yan RL, Tang D, Guo SB, Meng X, Chen BH (2012) Iodine-induced regioselective direct alkylation of azoles via in situ formed alkyliodide. Tetrahedron 68:7956–7959. https://doi.org/10.1016/j.tet.2012.07.008
Chen ZK, Wang BJ, Zhang JT, Yu WL, Liu ZX, Zhang YH (2015) Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org Chem Front 2:1107–1295. https://doi.org/10.1039/c5qo00004a
Cheng G-J, Song L-J, Yang Y-F, Zhang X, Wiest O, Wu Y-D (2013) Computational studies on the mechanism of the copper-catalyzed sp3–C–H cross-dehydrogenative coupling reaction. ChemPlusChem 78:943–951. https://doi.org/10.1002/cplu.201300117
Correia CA, Li CJ (2010) Copper-catalyzed cross-dehydrogenative coupling (CDC) of alkynes and benzylic C–H bonds. Adv Synth Catal 352:1446–1450. https://doi.org/10.1002/adsc.201000066
Cuthbertson JD, MacMillan DW (2015) The direct arylation of allylic sp(3) C–H bonds via organic and photoredox catalysis. Nature 519:74–77. https://doi.org/10.1038/nature14255
Cuttell DG, Kuang SM, Fanwick PE, McMillin DR, Walton RA (2002) Simple Cu(I) complexes with unprecedented excited-state lifetimes. J Am Chem Soc 124:6–7. https://doi.org/10.1021/ja012247h
Daugulis O, Do HQ, Shabashov D (2009) Palladium- and copper-catalyzed arylation of carbon-hydrogen bonds. Acc Chem Res 42:1074–1086. https://doi.org/10.1021/ar9000058
Deb ML, Borpatra PJ, Saikia PJ, Baruah PK (2017) Introducing tetramethylurea as a new methylene precursor: a microwave-assisted RuCl3-catalyzed cross dehydrogenative coupling approach to bis(indolyl)methanes. Org Biomol Chem 15:1435–1443. https://doi.org/10.1039/c6ob02671k
DeBoef B, Porter AL (2015) Aryl–aryl coupling via cross-dehydrogenative-coupling reactions. In: Li C-J (ed) From C–H to C–C bonds: cross-dehydrogenative-coupling. Green Chemistry Series, vol 26. The Royal Society of Chemistry, pp 114–132. https://doi.org/10.1039/9781782620082-00114
Deng G-J, Xiao F, Yang L (2015) Cross-dehydrogenative-coupling reactions involving allyl, benzyl and alkyl C–H bonds. In: Li C-J (ed) From C–H to C–C bonds: cross-dehydrogenative-coupling. Green Chemistry Series, vol 26. The Royal Society of Chemistry, pp 93–113. https://doi.org/10.1039/9781782620082-00093
Dhineshkumar J, Lamani M, Alagiri K, Prabhu KR (2013) A versatile C–H functionalization of tetrahydroisoquinolines catalyzed by iodine at aerobic conditions. Org Lett 15:1092–1095. https://doi.org/10.1021/ol4001153
Faisca Phillips AM, Pombeiro AJL (2018) Recent developments in transition metal-catalyzed cross-dehydrogenative coupling reactions of ethers and thioethers. ChemCatChem 10:3354–3383. https://doi.org/10.1002/cctc.201800582
Gao Q-H et al (2013) Metal-free dual sp3 C-H functionalization: I2-promoted domino oxidative cyclization to construct 2,5-disubstituted oxazoles. Tetrahedron 69:22–28. https://doi.org/10.1016/j.tet.2012.10.072
García Mancheño O, Stopka T (2013) TEMPO derivatives as alternative mild oxidants in carbon-carbon coupling reactions. Synthesis 45:1602–1611. https://doi.org/10.1055/s-0033-1338480
Ghosh I, Marzo L, Das A, Shaikh R, Konig B (2016) visible light mediated photoredox catalytic arylation reactions. Acc Chem Res 49:1566–1577. https://doi.org/10.1021/acs.accounts.6b00229
Ghosh S, Pahovnik D, Kragl U, Mejia E (2018) Isospecific copolymerization of cyclohexene oxide and carbon dioxide catalyzed by dialkylmagnesium compounds. Macromolecules 51:846–852. https://doi.org/10.1021/acs.macromol.7b02463
Girard SA, Knauber T, Li CJ (2014) The cross-dehydrogenative coupling of C(sp3)–H bonds: a versatile strategy for C–C bond formations. Angew Chem Int Ed Engl 53:74–100. https://doi.org/10.1002/anie.201304268
Guo X, Yu R, Li H, Li Z (2009) Iron-catalyzed tandem oxidative coupling and annulation: an efficient approach to construct polysubstituted benzofurans. J Am Chem Soc 131:17387–17393. https://doi.org/10.1021/ja907568j
Guo XX, Gu DW, Wu Z, Zhang W (2015) Copper-catalyzed C–H functionalization reactions: efficient synthesis of heterocycles. Chem Rev 115:1622–1651. https://doi.org/10.1021/cr500410y
He Z, Liu W, Li Z (2011) I2-catalyzed indole formation via oxidative cyclization of N-aryl enamines. Chem Asian J 6:1340–1343. https://doi.org/10.1002/asia.201100045
Hernandez-Perez AC, Caron A, Collins SK (2015) Photochemical synthesis of complex carbazoles: evaluation of electronic effects in both UV- and visible-light methods in continuous flow. Chemistry 21:16673–16678. https://doi.org/10.1002/chem.201502661
Hernandez-Perez AC, Collins SK (2013) A visible-light-mediated synthesis of carbazoles. Angew Chem Int Ed Engl 52:12696–12700. https://doi.org/10.1002/anie.201306920
Higgins RF et al (2016) Uncovering the roles of oxygen in Cr(III) photoredox catalysis. J Am Chem Soc 138:5451–5464. https://doi.org/10.1021/jacs.6b02723
Hirao T (1997) Vanadium in modern organic synthesis. Chem Rev 97:2707–2724. https://doi.org/10.1021/cr960014g
Hirao T (2007) Synthetic transformations via vanadium-induced redox reactions. In: Vanadium: the versatile metal, vol 974. ACS Symposium Series, vol 974. American Chemical Society, pp 2–27. https://doi.org/10.1021/bk-2007-0974.ch001
Hu W, Lin J-P, Song L-R, Long Y-Q (2015) Direct synthesis of 2-aryl-4-quinolones via transition-metal-free intramolecular oxidative C(sp3)–H/C(sp3)–H coupling. Org Lett 17:1268–1271. https://doi.org/10.1021/acs.orglett.5b00248
Huang HY, Wu HR, Wei F, Wang D, Liu L (2015) Iodine-catalyzed direct olefination of 2-oxindoles and alkenes via cross-dehydrogenative coupling (CDC) in air. Org Lett 17:3702–3705. https://doi.org/10.1021/acs.orglett.5b01662
Huo C, Chen F, Yuan Y, Xie H, Wang Y (2015) Iron catalyzed dual-oxidative dehydrogenative (DOD) tandem annulation of glycine derivatives with tetrahydrofurans. Org Lett 17:5028–5031. https://doi.org/10.1021/acs.orglett.5b02504
Hurst TE, Taylor RJK (2017) A Cu-catalysed radical cross-dehydrogenative coupling approach to acridanes and related heterocycles. Eur J Org Chem 2017:203–207. https://doi.org/10.1002/ejoc.201601336
Ito H, Ueda K, Itami K (2015) Cross-dehydrogenative-coupling reactions without metals. In: Li C-J (ed) From C–H to C–C bonds: cross-dehydrogenative-coupling. Green Chemistry Series, vol 26. The Royal Society of Chemistry, pp 153–196. https://doi.org/10.1039/9781782620082-00153
Jiang Y, Xu K, Zeng C (2018) Use of electrochemistry in the synthesis of heterocyclic structures. Chem Rev 118:4485–4540. https://doi.org/10.1021/acs.chemrev.7b00271
Jones KM, Klussmann M (2012) Oxidative coupling of tertiary amines: scope, mechanism and challenges. Synlett 2012:159–162. https://doi.org/10.1055/s-0031-1290117
Kang H et al (2017) Asymmetric oxidative coupling of phenols and hydroxycarbazoles. Org Lett 19:5505–5508. https://doi.org/10.1021/acs.orglett.7b02552
Kaswan P, Nandwana NK, DeBoef B, Kumar A (2016) Vanadyl acetylacetonate catalyzed methylenation of imidazo[1,2-a]pyridines by using dimethylacetamide as a methylene source: direct access to Bis(imidazo[1,2-a]pyridin-3-yl)methanes. Adv Synth Catal 358:2108–2115. https://doi.org/10.1002/adsc.201600225
Kaswan P, Porter A, Pericherla K, Simone M, Peters S, Kumar A, DeBoef B (2015) Oxidative cross-coupling of sp(3)- and sp(2)-hybridized C–H bonds: vanadium-catalyzed aminomethylation of imidazo[1,2-a]pyridines. Org Lett 17:5208–5211. https://doi.org/10.1021/acs.orglett.5b02539
Kern J-M, Sauvage J-P (1987) Photoassisted C–C coupling via electron transfer to benzylic halides by a bis(di-imine) copper(I) complex. J Chem Soc, Chem Commun: 546–548 https://doi.org/10.1039/c39870000546
Kim HY, Takizawa S, Sasai H, Oh K (2017) Reversal of enantioselectivity approach to BINOLs via single and dual 2-naphthol activation modes. Org Lett 19:3867–3870. https://doi.org/10.1021/acs.orglett.7b01734
Knorn M, Rawner T, Czerwieniec R, Reiser O (2015) [Copper(phenanthroline)(bisisonitrile)(+)-complexes for the visible-light-mediated atom transfer radical addition and allylation reactions. ACS Catal 5:5186–5193. https://doi.org/10.1021/acscatal.5b01071
Kshirsagar UA, Regev C, Parnes R, Pappo D (2013) Iron-catalyzed oxidative cross-coupling of phenols and alkenes. Org Lett 15:3174–3177. https://doi.org/10.1021/ol401532a
Kumar RA, Saidulu G, Prasad KR, Kumar GS, Sridhar B, Reddy KR (2012) Transition metal-free α-C(sp3)–H bond functionalization of amines by oxidative cross dehydrogenative coupling reaction: simple and direct access to C-4-alkylated 3,4-dihydroquinazoline derivatives. Adv Synth Catal 354:2985–2991. https://doi.org/10.1002/adsc.201200679
Laha JK, Jethava KP, Patel S (2015) Scope of successive C–H functionalizations of the methyl group in 3-picolines: intramolecular carbonylation of arenes to the metal-free synthesis of 4-azafluorenones. Org Lett 17:5890–5893. https://doi.org/10.1021/acs.orglett.5b03071
Langeslay RR, Kaphan DM, Marshall CL, Stair PC, Sattelberger AP, Delferro M (2018) Catalytic applications of vanadium: a mechanistic perspective. Chem Rev https://doi.org/10.1021/acs.chemrev.8b00245
Lao ZQ, Zhong WH, Lou QH, Li ZJ, Meng XB (2012) KI-catalyzed imidation of sp3 C–H bond adjacent to amide nitrogen atom. Org Biomol Chem 10:7869–7871. https://doi.org/10.1039/c2ob26430g
Larsen CB, Wenger OS (2018) Photoredox catalysis with metal complexes made from earth-abundant elements. Chem Eur J 24:2039–2058. https://doi.org/10.1002/chem.201703602
Lee A, Betori RC, Crane EA, Scheidt KA (2018) An enantioselective cross-dehydrogenative coupling catalysis approach to substituted tetrahydropyrans. J Am Chem Soc 140:6212–6216. https://doi.org/10.1021/jacs.8b03063
Lewis ES, Perry JM, Grinstein RH (1970) Mechanism of hydride transfer. III. Rates and isotope effects in the quinone oxidation of leuco triphenylmethane dyes. J Am Chem Soc 92:899–905. https://doi.org/10.1021/ja00707a027
Li K, You J (2016) Cascade oxidative coupling/cyclization: a gateway to 3-amino polysubstituted five-membered heterocycles. J Org Chem 81:2327–2339. https://doi.org/10.1021/acs.joc.5b02838
Li Z, Bohle DS, Li CJ (2006) Cu-catalyzed cross-dehydrogenative coupling: a versatile strategy for C–C bond formations via the oxidative activation of sp(3) C–H bonds. Proc Natl Acad Sci USA 103:8928–8933. https://doi.org/10.1073/pnas.0601687103
Li Z, Li CJ (2004) CuBr-catalyzed efficient alkynylation of sp3 C–H bonds adjacent to a nitrogen atom. J Am Chem Soc 126:11810–11811. https://doi.org/10.1021/ja0460763
Li Z, Li CJ (2005) Highly efficient copper-catalyzed nitro-Mannich type reaction: cross-dehydrogenative-coupling between sp3 C–H bond and sp3 C–H bond. J Am Chem Soc 127:3672–3673. https://doi.org/10.1021/ja050058j
Li Z, Li CJ (2006) Catalytic allylic alkylation via the cross-dehydrogenative-coupling reaction between allylic sp3 C–H and methylenic sp3 C–H bonds. J Am Chem Soc 128:56–57. https://doi.org/10.1021/ja056541b
Li Z, Yu R, Li H (2008) Iron-catalyzed C–C bond formation by direct functionalization of C–H bonds adjacent to heteroatoms. Angew Chem Int Ed Engl 47:7497–7500. https://doi.org/10.1002/anie.200802215
Lingamurthy M, Jagadeesh Y, Ramakrishna K, Rao BV (2016) DDQ-promoted benzylic/allylic sp(3) C–H activation for the stereoselective intramolecular C–N bond formation: applications to the total synthesis of (−)-codonopsinine, (+)-5-epi-codonopsinine, (+)-radicamine B, and (−)-codonopsinol. J Org Chem 81:1367–1377. https://doi.org/10.1021/acs.joc.5b02275
Lingayya R, Vellakkaran M, Nagaiah K, Nanubolu JB (2015) Ruthenium as a single catalyst for two steps: one-pot ruthenium(II)-catalyzed aerobic oxidative dehydrogenation of dihydroquinazolinones and cross-coupling/annulation to give N-fused polycyclic heteroarenes. Asian J Org Chem 4:462–469. https://doi.org/10.1002/ajoc.201500025
Liu D, Lei A (2015) Iodine-catalyzed oxidative coupling reactions utilizing C–H and X–H as nucleophiles. Chem Asian J 10:806–823. https://doi.org/10.1002/asia.201403248
Liu L, Carroll PJ, Kozlowski MC (2015) Vanadium-catalyzed regioselective oxidative coupling of 2-hydroxycarbazoles. Org Lett 17:508–511. https://doi.org/10.1021/ol503521b
Liu WQ et al (2017) Visible light promoted synthesis of indoles by single photosensitizer under aerobic conditions. Org Lett 19:3251–3254. https://doi.org/10.1021/acs.orglett.7b01367
Long H et al (2017) Regio- and diastereoselective cross-dehydrogenative coupling of tetrahydropyridines with 1,3-dicarbonyl compounds. Org Lett 19:2146–2149. https://doi.org/10.1021/acs.orglett.7b00787
Lou J, Wang Q, Wu K, Wu P, Yu Z (2017) Iron-catalyzed oxidative C–H functionalization of internal olefins for the synthesis of tetrasubstituted furans. Org Lett 19:3287–3290. https://doi.org/10.1021/acs.orglett.7b01431
Lu H, Yang Q, Zhou Y, Guo Y, Deng Z, Ding Q, Peng Y (2014) Cross-coupling/annulations of quinazolones with alkynes for access to fused polycyclic heteroarenes under mild conditions. Org Biomol Chem 12:758–764. https://doi.org/10.1039/c3ob41955j
Lv L, Li Z (2016) Fe-catalyzed cross-dehydrogenative coupling reactions. Top Curr Chem 374:38. https://doi.org/10.1007/s41061-016-0038-y
Maes J, Maes BUW (2016) Chapter five—a journey through metal-catalyzed CH functionalization of heterocycles: insights and trends. In: Scriven EFV, Ramsden CA (eds) Advances in heterocyclic chemistry, vol 120. Academic Press, pp 137–194. https://doi.org/10.1016/bs.aihch.2016.04.005
Maiti S, Achar TK, Mal P (2017) An organic intermolecular dehydrogenative annulation reaction. Org Lett 19:2006–2009. https://doi.org/10.1021/acs.orglett.7b00562
Maiti S, Mal P (2017) Dehydrogenative aromatic ring fusion for carbazole synthesis via C–C/C–N bond formation and alkyl migration. Org Lett 19:2454–2457. https://doi.org/10.1021/acs.orglett.7b01117
Marzo L, Pagire SK, Reiser O, Konig B (2018) Visible-light photocatalysis: does it make a difference in organic synthesis? Angew Chem Int Ed Engl 57:10034–10072. https://doi.org/10.1002/anie.201709766
McCusker JK, Walda KN, Dunn RC, Simon JD, Magde D, Hendrickson DN (1993) Subpicosecond 1MLCT -5T2 intersystem crossing of low-spin polypyridyl ferrous complexes. J Am Chem Soc 115:298–307. https://doi.org/10.1021/ja00054a043
Meng QY et al (2017) Identifying key intermediates generated in situ from Cu(II) salt-catalyzed C–H functionalization of aromatic amines under illumination. Sci Adv 3:e1700666. https://doi.org/10.1126/sciadv.1700666
Mitchell D, Cole KP, Pollock PM, Coppert DM, Burkholder TP, Clayton JR (2012) Development and a practical synthesis of the JAK2 inhibitor LY2784544. Org Process Res Dev 16:70–81. https://doi.org/10.1021/op200229j
Miyaura N, Suzuki A (1995) Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev 95:2457–2483. https://doi.org/10.1021/Cr00039a007
Moselage M, Li J, Ackermann L (2016) Cobalt-catalyzed C–H activation. ACS catal 6:498–525. https://doi.org/10.1021/acscatal.5b02344
Murahashi S, Komiya N, Terai H, Nakae T (2003) Aerobic ruthenium-catalyzed oxidative cyanation of tertiary amines with sodium cyanide. J Am Chem Soc 125:15312–15313. https://doi.org/10.1021/ja0390303
Murahashi S, Naota T, Yonemura K (1988) Ruthenium-catalyzed cytochrome-P-450 type oxidation of tertiary-amines with alkyl hydroperoxides. J Am Chem Soc 110:8256–8258. https://doi.org/10.1021/Ja00232a060
Murahashi SI, Naota T, Miyaguchi N, Nakato T (1992) Ruthenium-catalyzed oxidation of tertiary-amines with hydrogen-peroxide in the presence of methanol. Tetrahedron Lett 33:6991–6994. https://doi.org/10.1016/S0040-4039(00)60914-0
Narayan R, Manna S, Antonchick AP (2015) Hypervalent iodine(III) in direct carbon-hydrogen bond functionalization. Synlett 26:1785–1803. https://doi.org/10.1055/s-0034-1379912
Narayanam JM, Stephenson CR (2011) Visible light photoredox catalysis: applications in organic synthesis. Chem Soc Rev 40:102–113. https://doi.org/10.1039/b913880n
Narute S, Pappo D (2017) Iron phosphate catalyzed asymmetric cross-dehydrogenative coupling of 2-naphthols with beta-ketoesters. Org Lett 19:2917–2920. https://doi.org/10.1021/acs.orglett.7b01152
Nicholls TP, Constable GE, Robertson JC, Gardiner MG, Bissember AC (2015) Brønsted acid cocatalysis in copper(I)-photocatalyzed α-amino C–H bond functionalization. ACS Catal 6:451–457. https://doi.org/10.1021/acscatal.5b02014
Nobuta T, Tada N, Fujiya A, Kariya A, Miura T, Itoh A (2013) Molecular iodine catalyzed cross-dehydrogenative coupling reaction between two sp3 C–H bonds using hydrogen peroxide. Org Lett 15:574–577. https://doi.org/10.1021/ol303389t
Ohkubo K, Fujimoto A, Fukuzumi S (2013) Photocatalytic monofluorination of benzene by fluoride via photoinduced electron transfer with 3-cyano-1-methylquinolinium. The journal of physical chemistry A 117:10719–10725. https://doi.org/10.1021/jp408315a
Ohkubo K, Kobayashi T, Fukuzumi S (2011) Direct oxygenation of benzene to phenol using quinolinium ions as homogeneous photocatalysts. Angew Chem Int Ed Engl 50:8652–8655. https://doi.org/10.1002/anie.201102931
Otto S et al (2017) Photo-chromium: sensitizer for visible-light-induced oxidative C–H bond functionalization-electron or energy transfer? ChemPhotoChem 1:344–349. https://doi.org/10.1002/cptc.201700077
Pan B, Liu B, Yue E, Liu Q, Yang X, Wang Z, Sun W-H (2016) A ruthenium catalyst with unprecedented effectiveness for the coupling cyclization of γ-amino alcohols and secondary alcohols. ACS Catal 6:1247–1253. https://doi.org/10.1021/acscatal.5b02638
Pan J, Li X, Qiu X, Luo X, Jiao N (2018) Copper-catalyzed oxygenation approach to oxazoles from amines, alkynes, and molecular oxygen. Org Lett 20:2762–2765. https://doi.org/10.1021/acs.orglett.8b00992
Parisien-Collette S, Hernandez-Perez AC, Collins SK (2016) Photochemical synthesis of carbazoles using an [Fe(phen)3](NTf2)2/O2 catalyst system: catalysis toward sustainability. Org Lett 18:4994–4997. https://doi.org/10.1021/acs.orglett.6b02456
Parvatkar PT, Manetsch R, Banik BK (2018) Metal-free cross-dehydrogenative coupling (CDC): molecular iodine as a versatile catalyst/reagent for CDC reactions. Chem Asian J 0. https://doi.org/10.1002/asia.201801237
Parvatkar PT, Parameswaran PS, Tilve SG (2012) Recent developments in the synthesis of five- and six-membered heterocycles using molecular iodine. Chem Eur J 18:5460–5489. https://doi.org/10.1002/chem.201100324
Patil MR, Dedhia NP, Kapdi AR, Kumar AV (2018) Cobalt(II)/ N-hydroxyphthalimide-catalyzed cross-dehydrogenative coupling reaction at room temperature under aerobic condition. J Org Chem 83:4477–4490. https://doi.org/10.1021/acs.joc.8b00203
Peng F, Li LL, Liu J, Chen ZW (2018) Copper-catalyzed oxidative cross-coupling/C–C bond cleavage/cyclization of aryl methyl ketones with 4-aminocoumarins: domino synthesis of dicoumarin-fused [1,5]-diazocines. Asian J Org Chem 7:1667–1673. https://doi.org/10.1002/ajoc.201800306
Pirtsch M, Paria S, Matsuno T, Isobe H, Reiser O (2012) [Cu(dap)2Cl] as an efficient visible-light-driven photoredox catalyst in carbon-carbon bond-forming reactions. Chem Eur J 18:7336–7340. https://doi.org/10.1002/chem.201200967
Prier CK, Rankic DA, MacMillan DW (2013) Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem Rev 113:5322–5363. https://doi.org/10.1021/cr300503r
Ray D, Manikandan T, Roy A, Tripathi KN, Singh RP (2015) Ligand-promoted intramolecular dehydrogenative cross-coupling using a Cu catalyst: direct access to polycyclic heteroarenes. Chem Commun 51:7065–7068. https://doi.org/10.1039/c5cc01817j
Reddy NNK, Donthiri RR, Ravi C, Adimurthy S (2016) Iodine-catalyzed [3 + 2] cyclization of 2-pyridylesters and chalcones: metal-free approach for the synthesis of substituted indolizines. Tetrahedron Lett 57:3243–3246. https://doi.org/10.1016/j.tetlet.2016.05.083
Richter H, Garcia Mancheno O (2011) TEMPO oxoammonium salt-mediated dehydrogenative Povarov/oxidation tandem reaction of N-alkyl anilines. Org Lett 13:6066–6069. https://doi.org/10.1021/ol202552y
Romero NA, Margrey KA, Tay NE, Nicewicz DA (2015) Site-selective arene C–H amination via photoredox catalysis. Science 349:1326–1330. https://doi.org/10.1126/science.aac9895
Romero NA, Nicewicz DA (2016) Organic photoredox catalysis. Chem Rev 116:10075–10166. https://doi.org/10.1021/acs.chemrev.6b00057
Roslin S, Odell LR (2017) Visible-light photocatalysis as an enabling tool for the functionalization of unactivated C(sp3)-substrates. Eur J Org Chem 2017:1993–2007. https://doi.org/10.1002/ejoc.201601479
Roudesly F, Oble J, Poli G (2017) Metal-catalyzed CH activation/functionalization: the fundamentals. J Mol Catal A: Chem 426:275–296. https://doi.org/10.1016/j.molcata.2016.06.020
Sagadevan A, Ragupathi A, Hwang KC (2013) Visible-light-induced, copper(I)-catalysed C–N coupling between o-phenylenediamine and terminal alkynes: one-pot synthesis of 3-phenyl-2-hydroxy-quinoxalines. Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology 12:2110–2118. https://doi.org/10.1039/c3pp50186h
Sagadevan A, Ragupathi A, Hwang KC (2015) Photoinduced copper-catalyzed regioselective synthesis of indoles: three-component coupling of arylamines, terminal alkynes, and quinones. Angew Chem Int Ed Engl 54:13896–13901. https://doi.org/10.1002/anie.201506579
Sako M, Takeuchi Y, Tsujihara T, Kodera J, Kawano T, Takizawa S, Sasai H (2016) Efficient enantioselective synthesis of oxahelicenes using redox/acid cooperative catalysts. J Am Chem Soc 138:11481–11484. https://doi.org/10.1021/jacs.6b07424
Sako M, Takizawa S, Yoshida Y, Sasai H (2015) Enantioselective and aerobic oxidative coupling of 2-naphthol derivatives using chiral dinuclear vanadium(V) complex in water. Tetrahedron: Asymmetry 26:613–616 https://doi.org/10.1016/j.tetasy.2015.05.002
Sar D, Bag R, Yashmeen A, Bag SS, Punniyamurthy T (2015) Synthesis of functionalized pyrazoles via vanadium-catalyzed C–N dehydrogenative cross-coupling and fluorescence switch-on sensing of BSA protein. Org Lett 17:5308–5311. https://doi.org/10.1021/acs.orglett.5b02669
Schultz DM, Yoon TP (2014) Solar synthesis: prospects in visible light photocatalysis. Science 343:1239176. https://doi.org/10.1126/science.1239176
Sha Q, Arman H, Doyle MP (2015) Three-component cascade reactions with 2,3-Diketoesters: a novel metal-free synthesis of 5-vinyl-pyrrole and 4-hydroxy-indole derivatives. Org Lett 17:3876–3879. https://doi.org/10.1021/acs.orglett.5b01855
Sharma R, Abdullaha M, Bharate SB (2017) Oxidant-controlled C-sp(2)/sp(3)-H cross-dehydrogenative coupling of N-heterocycles with benzylamines. J Org Chem 82:9786–9793. https://doi.org/10.1021/acs.joc.7b00856
Shen Y, Li M, Wang S, Zhan T, Tan Z, Guo CC (2009) An efficient copper-catalyzed oxidative Mannich reaction between tertiary amines and methyl ketones. Chem Commun 953–955. https://doi.org/10.1039/b819657e
Shi X, Chen X, Wang M, Zhang X, Fan X (2018) Regioselective synthesis of acylated N-heterocycles via the cascade reactions of saturated cyclic amines with 2-Oxo-2-arylacetic acids. J Org Chem 83:6524–6533. https://doi.org/10.1021/acs.joc.8b00805
Shi X, Zhang F, Luo W-K, Yang L (2017) Oxidant-triggered C1-benzylation of isoquinoline by iodine-catalyzed cross-dehydrogenative-coupling with methylarenes. Synlett 13:494–498. https://doi.org/10.1055/s-0036-1588331
Shuai Q, Yang L, Guo X, Basle O, Li CJ (2010) Rhodium-catalyzed oxidative C–H arylation of 2-arylpyridine derivatives via decarbonylation of aromatic aldehydes. J Am Chem Soc 132:12212–12213. https://doi.org/10.1021/ja105396b
Shukla G, Srivastava A, Yadav D, Singh MS (2018) Copper-catalyzed one-pot cross-dehydrogenative thienannulation: chemoselective access to naphtho[2,1-b]thiophene-4,5-diones and subsequent transformation to benzo[a]thieno[3,2-c]phenazines. J Org Chem 83:2173–2181. https://doi.org/10.1021/acs.joc.7b03092
Singhal S, Jain SL, Sain B (2009) An efficient aerobic oxidative cyanation of tertiary amines with sodium cyanide using vanadium based systems as catalysts. Chem Commun 2371–2372. https://doi.org/10.1039/b820402k
Skubi KL, Blum TR, Yoon TP (2016) Dual catalysis strategies in photochemical synthesis. Chem Rev 116:10035–10074. https://doi.org/10.1021/acs.chemrev.6b00018
Song C, Dong X, Yi H, Chiang C-W, Lei A (2018) DDQ-catalyzed direct C(sp3)–H amination of alkylheteroarenes: synthesis of biheteroarenes under aerobic and metal-free conditions. ACS Catal 8:2195–2199. https://doi.org/10.1021/acscatal.7b04434
Srimani D, Ben-David Y, Milstein D (2013) Direct synthesis of pyridines and quinolines by coupling of gamma-amino-alcohols with secondary alcohols liberating H2 catalyzed by ruthenium pincer complexes. Chem Commun 49:6632–6634. https://doi.org/10.1039/c3cc43227k
Stevenson SM, Higgins RF, Shores MP, Ferreira EM (2017) Chromium photocatalysis: accessing structural complements to Diels-Alder adducts with electron-deficient dienophiles. Chem Sci 8:654–660. https://doi.org/10.1039/c6sc03303b
Stevenson SM, Shores MP, Ferreira EM (2015) Photooxidizing chromium catalysts for promoting radical cation cycloadditions. Angew Chem 127:6606–6610. https://doi.org/10.1002/ange.201501220
Stevenson SM, Shores MP, Ferreira EM (2015) Photooxidizing chromium catalysts for promoting radical cation cycloadditions. Angew Chem Int Ed Engl 54:6506–6510. https://doi.org/10.1002/anie.201501220
Sunil UT, Sushma SK, Satish AD, Swapnil RS, Rajendra PP (2012) Molecular iodine: an efficient and versatile reagent for organic synthesis. Curr Org Chem 16:1485–1501. https://doi.org/10.2174/138527212800672574
Suva Paria OR (2018) Visible light and copper complexes: a promising match in photoredox catalysis. In: Stephenson C, Yoon T, MacMillan DWC (eds) Visible light photocatalysis in organic chemistry. Wiely. https://doi.org/10.1002/9783527674145.ch7
Takizawa S, Kodera J, Yoshida Y, Sako M, Breukers S, Enders D, Sasai H (2014) Enantioselective oxidative-coupling of polycyclic phenols. Tetrahedron 70:1786–1793. https://doi.org/10.1016/j.tet.2014.01.017
Tan DW, Li HX, Zhu DL, Li HY, Young DJ, Yao JL, Lang JP (2018) Ligand-controlled copper(I)-catalyzed cross-coupling of secondary and primary alcohols to alpha-alkylated ketones, pyridines, and quinolines. Org Lett 20:608–611. https://doi.org/10.1021/acs.orglett.7b03726
Tan Z, Jiang H, Zhang M (2016) Ruthenium-catalyzed dehydrogenative beta-benzylation of 1,2,3,4-tetrahydroquinolines with aryl aldehydes: access to functionalized quinolines. Org Lett 18:3174–3177. https://doi.org/10.1021/acs.orglett.6b01390
Tang S, Liu K, Long Y, Gao X, Gao M, Lei A (2015) Iodine-catalyzed radical oxidative annulation for the construction of dihydrofurans and indolizines. Org Lett 17:2404–2407. https://doi.org/10.1021/acs.orglett.5b00912
Tang S, Liu K, Long Y, Qi X, Lan Y, Lei A (2015) Tuning radical reactivity using iodine in oxidative C(sp(3))-H/C(sp)-H cross-coupling: an easy way toward the synthesis of furans and indolizines. Chem Commun 51:8769–8772. https://doi.org/10.1039/c5cc01825k
Tanoue A, Yoo WJ, Kobayashi S (2014) Sulfuryl chloride as an efficient initiator for the metal-free aerobic cross-dehydrogenative coupling reaction of tertiary amines. Org Lett 16:2346–2349. https://doi.org/10.1021/ol500661t
Trost BM (1967) Dehydrogenation mechanisms. On the mechanism of dehydrogenation of acenaphthene by quinones. J Am Chem Soc 89:1847–1851. https://doi.org/10.1021/ja00984a017
Tsang ASK, Jensen P, Hook JM, Hashmi ASK, Todd MH (2011) An oxidative carbon–carbon bond-forming reaction proceeds via an isolable iminium ion. Pure Appl Chem 83:655–665. https://doi.org/10.1351/pac-con-11-01-01
Tsang ASK, Park SJ, Todd MH (2015) Mechanisms of cross-dehydrogenative-coupling reactions. In: Li C-J (ed) From C–H to C–C bonds: cross-dehydrogenative-coupling. RSC Green Chemistry Series, vol 26. The Royal Society of Chemistry, pp 254–294. https://doi.org/10.1039/9781782620082-00254
Tsang ASK, Todd MH (2009) Facile synthesis of vicinal diamines via oxidation of N-phenyltetrahydroisoquinolines with DDQ. Tetrahedron Lett 50:1199–1202. https://doi.org/10.1016/j.tetlet.2008.12.101
Twilton J, Le C, Zhang P, Shaw MH, Evans RW, MacMillan DWC (2017) The merger of transition metal and photocatalysis. Nat Rev Chem 1:0052. https://doi.org/10.1038/S41570-017-0052
Ueda H, Yoshida K, Tokuyama H (2014) Acetic acid promoted metal-free aerobic carbon-carbon bond forming reactions at alpha-position of tertiary amines. Org Lett 16:4194–4197. https://doi.org/10.1021/ol5018883
Vuppalapati SVN, Lee YR (2012) Iodine-catalyzed efficient synthesis of azaarene substituted 3-hydroxy-2-oxindole derivatives through sp3 C–H functionalization. Tetrahedron 68:8286–8292. https://doi.org/10.1016/j.tet.2012.07.051
Waghmode NA, Kalbandhe AH, Thorat PB, Karade NN (2016) Metal-free new synthesis of 1,3-naphthoxazines via intramolecular cross dehydrogenative-coupling reaction of 1-(α-aminoalkyl)-2-naphthols using hypervalent iodine(III) reagent. Tetrahedron Lett 57:680–683. https://doi.org/10.1016/j.tetlet.2015.12.117
Wang B et al (2015) Long-lived excited states of zwitterionic copper(I) complexes for photoinduced cross-dehydrogenative coupling reactions. Chem Eur J 21:1184–1190. https://doi.org/10.1002/chem.201405356
Wang CS, Dixneuf PH, Soule JF (2018) Photoredox catalysis for building C–C bonds from C(sp(2))-H bonds. Chem Rev 118:7532–7585. https://doi.org/10.1021/acs.chemrev.8b00077
Wang L, Ackermann L (2013) Versatile pyrrole synthesis through ruthenium(II)-catalyzed alkene C–H bond functionalization on enamines. Org Lett 15:176–179. https://doi.org/10.1021/ol303224e
Waters WA (1946) Evidence for the dehydrogenation theory of oxidation. Trans Faraday Soc 42:184–190. https://doi.org/10.1039/TF9464200184
Wendlandt AE, Suess AM, Stahl SS (2011) Copper-catalyzed aerobic oxidative C–H functionalizations: trends and mechanistic insights. Angew Chem Int Ed Engl 50:11062–11087. https://doi.org/10.1002/anie.201103945
Wenger OS (2018) Photoactive complexes with earth-abundant metals. J Am Chem Soc https://doi.org/10.1021/jacs.8b08822
Wu CJ, Zhong JJ, Meng QY, Lei T, Gao XW, Tung CH, Wu LZ (2015) Cobalt-catalyzed cross-dehydrogenative coupling reaction in water by visible light. Org Lett 17:884–887. https://doi.org/10.1021/ol503744a
Wu X-F, Beller M (2014a) Cobalt-catalyzed heterocycle synthesis. In: Economic synthesis of heterocycles: zinc, iron, copper, cobalt, manganese and nickel catalysts. RSC Catalysis Series. The Royal Society of Chemistry, pp 349–385. https://doi.org/10.1039/9781782620839-00349
Wu X-F, Beller M (2014b) Copper-catalyzed heterocycle synthesis. In: Economic synthesis of heterocycles: zinc, iron, copper, cobalt, manganese and nickel catalysts. RSC Catalysis Series. The Royal Society of Chemistry, pp 159–348. https://doi.org/10.1039/9781782620839-00159
Wu X-F, Beller M (2014c) Iron-catalyzed heterocycle synthesis. In: Economic synthesis of heterocycles: zinc, iron, copper, cobalt, manganese and nickel catalysts. RSC Catalysis Series. The Royal Society of Chemistry, pp 59–158. https://doi.org/10.1039/9781782620839-00059
Wu X, Zhao P, Geng X, Wang C, Wu YD, Wu AX (2018) Synthesis of pyrrole-2-carbaldehyde derivatives by oxidative annulation and direct Csp3–H to C=O oxidation. Org Lett 20:688–691. https://doi.org/10.1021/acs.orglett.7b03821
Wu Y, Arenas I, Broomfield LM, Martin E, Shafir A (2015) Hypervalent activation as a key step for dehydrogenative ortho C–C coupling of iodoarenes. Chem Eur J 21:18779–18784. https://doi.org/10.1002/chem.201503987
Xiang L et al (2014) I2-mediated oxidative cyclization for synthesis of substituted indolizines. J Org Chem 79:10641–10647. https://doi.org/10.1021/jo5019574
Xiang M, Meng Q-Y, Gao X-W, Lei T, Chen B, Tung C-H, Wu L-Z (2016) Reactivity and mechanistic insight into the cross coupling reaction between isochromans and β-keto esters through C–H bond activation under visible light irradiation. Org Chem Front 3:486–490. https://doi.org/10.1039/C5QO00412H
Xie J, Huang Y, Song H, Liu Y, Wang Q (2017) Copper-catalyzed aerobic oxidative [2 + 3] cyclization/aromatization cascade reaction: atom-economical access to tetrasubstituted 4,5-Biscarbonyl Imidazoles. Org Lett 19:6056–6059. https://doi.org/10.1021/acs.orglett.7b02767
Xie Z, Zan X, Sun S, Pan X, Liu L (2016) Organocatalytic enantioselective cross-dehydrogenative coupling of N-carbamoyl cyclic amines with aldehydes. Org Lett 18:3944–3947. https://doi.org/10.1021/acs.orglett.6b01625
Xing Y, Wang N-X, Zhang W (2015) Advances in transition-metal-catalyzed direct sp3-carbon–hydrogen bond functionalization. Synlett 26:2088–2098. https://doi.org/10.1055/s-0034-1381031
Xue W-j, Gao Q-H, Wu A-x (2015) Molecular iodine mediated oxidative cross-coupling of sp3 C–H with sp2 C–H: direct synthesis of substituted indolo[2,3-b]carbazoles via formal [2 + 2+1 + 1] cyclization. Tetrahedron Lett 56:7115–7119. https://doi.org/10.1016/j.tetlet.2015.11.026
Xue XS, Ji P, Zhou B, Cheng JP (2017) The essential role of bond energetics in C–H activation/functionalization. Chem Rev 117:8622–8648. https://doi.org/10.1021/acs.chemrev.6b00664
Yang Q, Li S, Wang J (2018) Cobalt-catalyzed cross-dehydrogenative coupling of imidazo[1,2-a]pyridines with isochroman using molecular oxygen as the oxidant. Org Chem Front 5:577–581. https://doi.org/10.1039/C7QO00875A
Yang Y, Lan J, You J (2017) Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem Rev 117:8787–8863. https://doi.org/10.1021/acs.chemrev.6b00567
Yavari I, Hosseinpour R, Skoulika S (2015) Iodine-mediated diastereoselective cyclopropanation of arylidene malononotriles by 2,6-dimethylquinoline. Synlett 26:380–384. https://doi.org/10.1055/s-0034-1379496
Yoon TP, Ischay MA, Du J (2010) Visible light photocatalysis as a greener approach to photochemical synthesis. Nat Chem 2:527–532. https://doi.org/10.1038/nchem.687
Yu JB, Zhang Y, Jiang ZJ, Su WK (2016) Mechanically induced Fe(III) catalysis at room temperature: solvent-free cross-dehydrogenative coupling of 3-benzylic indoles with methylenes/indoles. J Org Chem 81:11514–11520. https://doi.org/10.1021/acs.joc.6b02197
Zhang B, Cui Y, Jiao N (2012) Metal-free TEMPO-catalyzed oxidative C–C bond formation from Csp3–H bonds using molecular oxygen as the oxidant. Chem Commun 48:4498–4500. https://doi.org/10.1039/c2cc30684k
Zhang C, Li TL, Wang LG, Rao Y (2017) Synthesis of diverse heterocycles via one-pot cascade cross-dehydrogenative-coupling (CDC)/cyclization reaction. Org Chem Front 4:386–391. https://doi.org/10.1039/c6qo00522e
Zhang C, Tang C, Jiao N (2012) Recent advances in copper-catalyzed dehydrogenative functionalization via a single electron transfer (SET) process. Chem Soc Rev 41:3464–3484. https://doi.org/10.1039/c2cs15323h
Zhang R, Qin Y, Zhang L, Luo S (2017) Oxidative synthesis of benzimidazoles, quinoxalines, and benzoxazoles from primary amines by ortho-quinone catalysis. Org Lett 19:5629–5632. https://doi.org/10.1021/acs.orglett.7b02786
Zhang Y, Schulz M, Wachtler M, Karnahl M, Dietzek B (2018) Heteroleptic diimine-diphosphine Cu(I) complexes as an alternative towards noble-metal based photosensitizers: design strategies, photophysical properties and perspective applications. Coord Chem Rev 356:127–146. https://doi.org/10.1016/j.ccr.2017.10.016
Zhang Z, Pi C, Tong H, Cui X, Wu Y (2017) Iodine-catalyzed direct C–H alkenylation of azaheterocycle N-oxides with alkenes. Org Lett 19:440–443. https://doi.org/10.1021/acs.orglett.6b03399
Zhao J, Fang H, Qian P, Han J, Pan Y (2014) Metal-free oxidative C(sp3)–H bond functionalization of alkanes and conjugate addition to chromones. Org Lett 16:5342–5345. https://doi.org/10.1021/ol502524d
Zhao M-N, Yu L, Hui R-R, Ren Z-H, Wang Y-Y, Guan Z-H (2016) Iron-catalyzed dehydrogenative [4 + 2] cycloaddition of tertiary anilines and enamides for the synthesis of tetrahydroquinolines with amido-substituted quaternary carbon centers. ACS Catal 6:3473–3477. https://doi.org/10.1021/acscatal.6b00849
Zhao X, Liu T-X, Ma N, Zhang G (2017) In situ generated TEMPO oxoammonium salt mediated tandem cyclization of β-oxoamides with amine hydrochlorides for the synthesis of pyrrolin-4-ones. The J Org Chem 82:6125–6132. https://doi.org/10.1021/acs.joc.7b00686
Zhu Z-Q, Xiao L-J, Zhou C-C, Song H-L, Xie Z-B, Le Z-G (2018) A visible-light-promoted cross-dehydrogenative-coupling reaction of N-arylglycine esters with imidazo[1,2-a]pyridines. Tetrahedron Lett 59:3326–3331. https://doi.org/10.1016/j.tetlet.2018.07.047
Zou YQ, Lu LQ, Fu L, Chang NJ, Rong J, Chen JR, Xiao WJ (2011) Visible-light-induced oxidation/[3 + 2] cycloaddition/oxidative aromatization sequence: a photocatalytic strategy to construct pyrrolo[2,1-a]isoquinolines. Angew Chem Int Ed Engl 50:7171–7175. https://doi.org/10.1002/anie.201102306
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Almasalma, A.A., Mejía, E. (2019). Mechanistic Pathways Toward the Synthesis of Heterocycles Under Cross-Dehydrogenative Conditions. In: Srivastava, A., Jana, C. (eds) Heterocycles via Cross Dehydrogenative Coupling. Springer, Singapore. https://doi.org/10.1007/978-981-13-9144-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-9144-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9143-9
Online ISBN: 978-981-13-9144-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)