Abstract
The global burden of dengue is increasing at an alarming rate and increased international travel will lead to constant importation of dengue virus into non-endemic areas. The potential for dengue epidemics in such countries during seasons with permissive temperatures has already been underlined by epidemics in Japan and Madeira. While improved surveillance can help identify clinical cases of dengue, differentiating between imported and autochthonous cases remains problematical. Implementation of a threshold criterion can help in identifying aberrant incidences of dengue. This threshold approach was applied to dengue cases reported in the Japanese surveillance system from 2011–2019. Several aberrant incidences occurring during consecutive weeks were detected, one of which was concomitant to the Yoyogi Park Tokyo epidemic but in another area, Kanagawa, and another above threshold week was coincidental with a symptomatic case of a German traveller. This indicates autochthonous transmission. Despite the occurrence of several alert periods, however, on no occasion did the spread of dengue progress into a full epidemic as was seen in Yoyogi. It thus seems likely that Yoyogi Park was a particular event and that stochastic die-out of viruses is occurring frequently without progression, perhaps reflecting the negative impact of societal infra-structure on dengue transmission despite permissive temperatures. Implementation of a dengue epidemic threshold as used for seasonal influenza may provide a basis for future seroprevalence studies to assess the true prevalence of dengue in light of the high frequency of subclinical, asymptomatic infections.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Dengue is a mosquito-borne viral disease with a major international public health concern as a consequence of urbanization, globalization, and global warming (Gubler and Kuno 1997; Halstead 2007; Lambrechts et al. 2010; WHO 2015). Dengue outbreaks have become more frequent in recent years. More than 3.5 billion people are at risk of dengue virus (DENV) infection, and it has recently been estimated that there are 390 million DENV infections every year, of which up to three-quarters are estimated to be subclinical, resulting in insufficient discomfort for clinical consultation (Bhatt et al. 2013; Grange et al. 2014). However, infection can lead to dengue haemorrhagic fever (DHF) and dengue shock syndrome leading to an estimated 20,000 deaths annually. Over the past few decades, the number of countries affected worldwide and the frequency of epidemics have increased significantly (WHO 2015). This prolific increase has been associated with increased global trade and travel, as well as societal changes such as population growth and increasing urbanization, particularly in tropical cities with poor waste and water management. This leads to the proliferation of the major mosquito species that transmit DENV, Aedes aegypti and Ae. albopictus (Gubler 2002). Aedes aegypti is restricted to the tropics and subtropics and is well adapted to the peridomestic environment. Aedes albopictus, by contrast, spread from southeast Asia and has successfully invaded temperate regions such as southern Europe and Japan and found in more sub-urban or rural areas, and in outdoor habitats, such as gardens, parks, or forests. In Japan, Ae. albopictus is the main vector, although larvae and pupae of Ae. aegypti have been intermittently detected at international airports in Japan since 2012 (Sukehiro et al. 2013).
Rising temperatures associated with global climate change are enabling the spread and establishment of many disease vector species and will influence the capacity of mosquito populations to transmit arthropod-borne viruses such as DENV. Warming can generate optimal temperatures for decreasing the extrinsic incubation period of viral development within the mosquito and mosquito survival, growth, and biting rates (Hales et al. 2002; Misslin et al. 2016; Quam et al. 2016; Thai and Anders 2011). International travel will ensure importation of virus into non-endemic countries from regions endemic for dengue (Chen and Wilson 2008) and the increased global burden of dengue will have knock-on effects in such non-endemic countries. The potential threat of DENV invasion into naïve areas is illustrated by cases of autochthonous dengue intermittently occurring in France and the US over the past decade (Effler et al. 2005; La Ruche et al. 2010; Murray et al. 2013) and both Madeira, Portugal and Tokyo, Japan have recently experienced unprecedented epidemics (Alves et al. 2013; Kutsuna et al. 2015; Seki et al. 2015). In so far as the majority of DENV infections are subclinical and infected individuals can infect mosquitoes prior to expressing symptoms, or even if experiencing no clinical symptoms (Duong et al. 2015), repeated “silent” DENV invasion is likely to be increasingly frequent.
Within this context we examine the case of Japan, which had an unprecedented autochthonous dengue 1 epidemic in Tokyo in 2014, and which is experiencing an ever-increasing number of dengue cases every year, thereby providing an increasing opportunity for new epidemics. Analysing dengue case surveillance data over the last ten years and for the specific case of the Yoyogi Park, Tokyo epidemic, we assess whether (1) there were particular prefectures with constant dengue cases, (2) there were incidences (other than the Yoyogi Park epidemic) when the number of dengue cases exceeded that expected due to importation and whether these incidences represented potential foci of epidemics. Finally, we analyze the Yoyogi epidemic, discuss whether it was an unfortunate rare event, the probability of a repeat epidemic in the near future and the value of establishing a threshold for dengue alert.
2 Materials and Methods
2.1 Surveillance System and Case Definition of Dengue Fever
Since 1999, dengue has been one of the notifiable diseases under surveillance across Japan. The case definition of dengue fever requires suspicious clinical symptoms (fever, myalgia, arthralgia, retro-orbital pain, rash, thrombocytopenia, and leukopenia) and laboratory confirmation (virus isolation, RT-PCR, detection of NS1 antigen or anti-dengue IgM antibodies, hemagglutination inhibition test or neutralization test) (Ministry of Health, Labour and Welfare 2011). Among these diagnostics, the rapid diagnostic test (NS1 ELISA test) has been covered by health insurance for the hospitalized cases after the 2014 epidemic.
In the surveillance system (Fig. 1), physicians report clinically suspect dengue cases immediately to the health centre via fax with demographic, clinical, exposure, and travel history information (Taniguchi et al. 2008) The public health centre is the primary level public health institution (PHI) established in all prefectures and many government ordinance cities. The health centres immediately register the reported cases in the national electronic database and the case report is validated by the regional infectious disease surveillance centre (IDSC) or the regional health department as a substitute. The confirmed data becomes available throughout governmental institutions and other institutions related to public health via the database. The central institution of this surveillance is the National IDSC. Regional IDSC serves the same role at the regional level (Ministry of Health, Labour and Welfare 2018). It takes approximately two days for a case report to be transmitted from a doctor to the national IDSC. If there are no dengue diagnostics available in the hospital, the doctor submits the specimen to the prefectural PHI or National Institute of Infectious Diseases to test the specimen.
Key: NIID: National Institute of Infectious Diseases, IDSC: infectious disease surveillance centre under the institute, PHI: public health institutes.
2.2 Autochthonous Case Definition and Epidemic Response
The definition of an imported case is infection with travel to a dengue-infected area within two weeks prior to onset of symptoms and an autochthonous dengue case is otherwise (Tuberculosis and Infectious Disease Control Division, Health Service Bureau, Ministry of Health, Labour and Welfare 2016). After an autochthonous case is reported to the nearby health centre by the doctor, the health centre questions not only the patient but also the companions and the cohabitants about their activities and experience of mosquito bites from 14 days prior to onset of symptoms, in order to estimate the place of infection to implement active surveillance and intervention. On receiving the case report and the estimated place of infection, the regional municipalities implement insecticide treatment in the locality, with mosquito abundance measured prior to and after treatment; insecticide treatment may be repeated depending on the efficacy of the first treatment. In addition, environmental hygiene measures are recommended to the local population, as well use of insect repellents and community-based source reduction.
2.3 Dengue Data Source
All of the dengue cases diagnosed by doctors in Japan are registered in the database of surveillance system in Japan, which is published weekly at the website of Infectious Disease Weekly Report (IDWR), the Japanese Ministry of Health, Labour and Welfare surveillance system (National Institute of Infectious Diseases 2021b: www.nih.go.jp/niid/ja/idwr.html). The data are updated in the annual report of IDWR, which is available from 1999 to 2019 (National Institute of Infectious Diseases 2021a: www.niid.go.jp/niid/ja/allarticles/surveillance/2270-idwr/nenpou/10115-idwr-nenpo2019.html). With this data we describe the annual occurrence of dengue. In addition, we then calculate the critical community size (CCS). This is generally used as a broad indicator of the population size necessary for a pathogen to persist in the face of immunity and stochastic die-out (Teissier et al. 2020). That is, if the population size estimated to be necessary to maintain DENV permanently exceeds that of any prefecture, then the virus must be continually imported. The ambient temperature in Japan is suitable (>15 °C) for viral dissemination and spread by Ae. albopictus mosquitoes from May until October. Thus, we divided the annual data into two time periods, November–April and May–October, denoted hereafter cold and warm seasons. To estimate the CCS, the number of dengue-free four-week periods per year was plotted against population size and compared in cold and warm seasons (Tokyo epidemic autochthonous cases were removed prior to analyses). Population data were taken from the national census in 2015 (Statistics Bureau, Ministry of Internal Affairs and Communications 2017). A period of four weeks was considered to cover the lifecycle of the virus, including the incubation periods in both man and mosquito (on average respectively six and 15 days at 25 °C (Chan and Johansson 2012). Each year was thus divided into 13 periods by four weeks.
2.4 Background Threshold for Identifying Outliers
The largest metropolitan area, Greater Tokyo area, was then selected for study, encompassing the largest number of dengue cases across the study period. Greater Tokyo area consists of Tokyo, Saitama, Kanagawa, and Chiba. Since this area functions as a commuting zone, the area-based data were calculated from the sum of the case number of member prefectures in each week. To calculate the threshold, the data were extracted from the annual reports from 2005 to the end of 2019, including information on reporting prefectures and estimated place of infection. Tukey’s Box Plot method was used to establish the median background weekly incidence of dengue in each study area using the past six years’ data. The weekly threshold was defined as rounded-up value of the third quartile plus 1.5 × the interquartile range of the case numbers from the same week (using the previous six years prior to the year of comparison). A six-year period was chosen since it was the minimal required number of data points for calculating the box plot method (Senda et al. 2018). The six-previous-year weekly thresholds of cases in each prefecture were respectively applied to the weekly reported cases from 2011 to 2019. The Yoyogi Park outbreak was excluded from the analysis. We defined an outlier as a week with more than one case above or equal to the threshold. The “autochthonous epidemic alert” was defined as at least two consecutive weeks of outliers.
2.5 Outlying Events in the Tokyo Epidemic
The Tokyo epidemic in and around Yoyogi Park was analysed separately to assess whether the epidemic was a single event dominated by the cases from Yoyogi Park or whether the cases in neighbouring parks represented a second series of events. Non-linear (Gaussian, double Gaussian) regression models were fitted to the incidence data by day and by place (Yoyogi Park and/or other satellite parks) and the goodness of fit was assessed by the percent of variation explained.
For all statistical analyses and model fitting, we used Genstat version 15 (GenStat for Windows 15th Edition).
3 Results
3.1 Japan Annual and Monthly Incidence
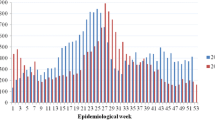
The number of imported cases has been rising steadily over the last decade (Fig. 2), concomitant with the increase in visitors, most especially from Korea, China, Taiwan, southeast Asian countries. Until 2015, the number of outbound Japanese travellers exceeded the inbound foreign travellers, of which a third went to dengue endemic countries (including China) (JTB Tourism Research & Consulting Co. 2021). However, since that period the number of incoming visitors has surpassed the outbound travellers. Although the number of tourists visiting Japan does increase marginally during the warm season, outbound Japanese travellers specifically those visiting dengue-endemic countries (for example, the Philippines, Thailand, and Singapore) peak in August. As can be seen in Fig. 3, peak reported cases occur during the summer months, notably August–October, which coincides with the dengue season in endemic countries in Asia.
3.2 Critical Community Size for Persistent Dengue
As shown in Fig. 4a, b, there is a negative relationship in both seasons between population size of the prefecture and the number of four-week periods during that season with no dengue cases occurring. The cold season relationship clearly reflects the baseline occurrence of imported dengue; during the cold season, the estimated CCS (13.5 million 95% confidence intervals 12.7–14.2 million) is larger than any single prefecture in Japan, indicating that there is no single area having a sufficient frequency of imported cases to maintain permanent, potentially circulating virus; the virus just dies out. However, by contrast, it is notable that the CCS during the warm season is significantly lower (11.8 million 95% confidence intervals 11.1–12.4 million) and the Tokyo prefecture is large enough to maintain transmission (13.5 million). On only four occasions did Tokyo report a dengue-free month during either the warm and cold seasons from 2010 until 2019. While this could be due to higher importation rates in Tokyo during the warm season, DENV is permanently circulating and could generate autochthonous infections. In addition, several smaller prefectures also show some years with dengue every month during the warm season. Overall, there was a three-fold decrease in the probability of having a dengue-free month anywhere in Japan than during the cold season (logistic regression of dengue free month over total months taking into account population size and year: odds ratio = 3.20, 95% confidence intervals 2.69–3.82, F1,904 = 170.4 P < 0.001). Thus, while imported cases are the likely cause of the majority of cases registered, it is not unfeasible that there is potential misdiagnosis of imported for autochthonous cases, where imported cases are defined simply on the basis of recent travel abroad. In the following section we extend a method we previously applied to attempting to identify potential situations of autochthonous transmission (Senda et al. 2018).
3.3 Dengue Incidence and Thresholds
In the Greater Tokyo area, and prefectures within this “commuting zone”, the threshold weekly incidence rate was calculated using weekly dengue incidence over the previous six years for each year from 2011 to 2019 using the box-plot method. The observed weekly incidence of dengue cases was then compared to the threshold and outliers were classified as those greater than the threshold by at least one dengue case. Such outlying dengue case weeks were detected in all prefectures and in the aggregated Greater area several times during the nine-year period (Fig. 3). There were several occasions when there were outliers for two consecutive weeks, our definition of an alert, occurring 14 times in the Greater Tokyo area: in 2012 (weeks 10–11 and 36–37), 2013 (weeks 19–20), 2015 (weeks 2–3), in 2016 (weeks 1–2, 12–14, 17–19, 28–29), in 2017 (weeks 3–4), and in 2019 (weeks 2–3, 18–20, 30–31, 39–41, 43–44 and 46–47) (Fig. 3). At the prefectural level, an alert was detected from August 25 to September 7, 2014 in Kanagawa, which coincides with the Tokyo autochthonous epidemic. In addition, nine “alerts” were identified in Tokyo: in 2013 (weeks 32–33), 2016 (weeks 13–14), 2017 (weeks 31–32), and 2019 (weeks 2–3, weeks 18–19, 30–31, 40–41, 43–44, 46–47). The alert in 2013 in Tokyo prefecture was coincidental with the period of visit by a German traveller who was allegedly infected with DENV in Japan (Schmidt-Chanasit et al. 2014). The Tokyo 2016 alerts occurred during (up to week 17) or just after the cold season and were due to an increase in the number of cases imported from Indonesia according to the National IDSC (National Institute of Infectious Diseases 2021c: Notification Trends Among Imported Dengue Cases in Japan). Most alarmingly, we detected five warm season alerts in 2019, three of which occurred almost sequentially during the period from weeks 39–47 (October and November). These alerts occurred just after the peak in imported cases from southeast Asia and elsewhere that occurred in August and September and that diminished steadily in October to reach background levels in November (National Institute of Infectious Diseases 2021c: Notification Trends Among Imported Dengue Cases in Japan). This would raise the suspicion of autochthonous transmission in autumn seeded by imported cases in the summer.
3.4 Tokyo Epidemic Yoyogi Park
The autochthonous 2014 Tokyo epidemic generated 162 confirmed dengue cases, which were spatially clustered around Yoyogi Park. Out of the 162 cases, 131 could be localized to a probable location of infection: namely 110 in Yoyogi Park and 21 distributed among four satellite parks as shown in Fig. 5a. Figure 5a also shows Shinjuku Gyoen Park, which although was not associated with any dengue cases, did have DENV-1 positive adult mosquitoes collected from September 11–25 after its closure on the 7th (Seki et al. 2015). To ascertain whether the cases in the satellite parks were simultaneous or subsequent to the Yoyogi cases, we fitted the following non-linear regression models: (1) Gaussian fit to all data from both parks; (2) a Double-Gaussian to all data from both parks; and (3) Gaussian with Yoyogi and satellite parks treated as independent. The double Gaussian curve yielded the poorest fit (F5,55 = 21.4 P < 0.001; 62.9% variation explained). The Gaussian fitted to all data yielded a similar result (F3,57 = 35.4 P < 0.001; 63.2% variation explained) (Fig. 5b). Treating parks as separate entities yielded the best fit (F5,116 = 45.9 P < 0.001; 65.0% variation explained) (Fig. 5c). The epidemics in Yoyogi and the satellite parks are therefore not contiguous, with the seeding event clearly arising in Yoyogi Park and leading to delayed cases in the satellite parks, but which failed to generate a large number of cases in any of the satellite parks.
Yoyogi Park Epidemic. a Map of parks with dengue cases: (1) Yoyogi Park, (2) Shinjuku Central Park, (3) Sotobori Park, (4) Ueno Park, (5) Meiji Jingu Gaien Park, ((6) Shinjuku Gyoen Park where only infected mosquitos were detected.) Map edited from electronic topographic map provided by Geospatial Information Authority of Japan to include all parks with cases in Tokyo epidemic (Geospatial Information Authority of Japan 2021); b Gaussian fit to all dengue case data by day and c Gaussian curve fit treating Yoyogi (red triangles) and satellite (green points) parks separately
4 Discussion
In this study we have addressed the potentially increasing invasion probability of dengue into Japan following the wake-up call of the 2014 Tokyo mini-epidemic. The number of dengue cases reported in Japan has been increasing yearly, is concomitant with the increasing human flux between Japan and dengue endemic areas, and related to the population size of the cities within Japan. Tokyo in particular has persistent cases of dengue throughout the warm months of the year and the frequency of anomalously warm spring, summer, and autumn months has been suggested to increase the probability of a second dengue epidemic in Japan at this time (Quam et al. 2016). Japan previously had epidemics in the 1940s, but not until 2014 when there were autochthonous cases reported. However, there have been several reports of travellers contracting dengue while visiting Japan, which suggests that DENV is circulating either as silent infections and/or that allegedly imported cases are indeed autochthonous (Kojima 2015; Nakayama et al. 2016; Schmidt-Chanasit et al. 2014). While acknowledging the persistent “background” rate of likely imported dengue cases, there are clear occasions when there are aberrantly high numbers of cases, supporting a role of autochthonous transmission, but which does not then lead to an epidemic, despite permissive conditions.
The critical population size analysis clearly showed that DENV can be expected to be circulating persistently throughout the warm season in Tokyo, whether from viral importation through returning tourists or visitors, or subsequent autochthonous transmission. Epidemic alerts based on geographical clustering are useful in targeting the potential reservoir (Stoddard et al. 2014), as was carried out for the Yoyogi Park epidemic, but will, as elsewhere, likely be too late in stopping the spread of the virus beyond the site (Reiter and Gubler 1997). Indeed, viral spread from Yoyogi Park was confirmed in the satellites park with cases and in Shinjuku Gyoen Park in adult mosquitos and may have been implicated in a dengue case in an international student residing adjacent to Yoyogi Park and falling ill upon return to the UK (Kojima 2015).
Diagnosis of imported dengue cases based on a recent history of travel may be misleading. For example, a dengue patient in Hyogo during the 2016 epidemic had stayed in Malaysia for 12–14 days before onset of symptoms, but also had recollection of mosquito bites six days before onset in Hyogo and the virus strain 100% matched with the Yoyogi strain. Unusual above-threshold incidences of dengue may provide an additional criterion for differentiation. Although no official epidemic occurred during the time of disease onset of the German traveller (Schmidt-Chanasit et al. 2014), our alert estimation based on the baseline threshold method did pinpoint to this period as being aberrant. Importantly, while unusually high “above-threshold” dengue incidences were noted during several time periods, there was never any subsequent progression into an epidemic, although the lengthy period above the alert threshold in 2019 is concerning. The recurrent conclusion from our estimations and observations, including the failure of the satellite parks near to Yoyogi Park and Shinjuku Gyoen to progress into comparable epidemics, is that there is substantial stochastic die-out of circulating DENV, despite permissive temperatures. Thus, while there may be significant viral import and climatic conditions highly permissive to transmission, societal factors may be overwhelmingly important and significantly have a negative impact upon viral spread through the population. This notwithstanding, the majority of infections, especially being primary infections, will likely go unnoticed and that the actual spread of DENV may be greater than we realize from surveillance data. Indeed, active serological and behavioural studies were conducted by the NIID on the 375 individuals who frequented Yoyogi Park and its vicinity (National Institute of Infectious Diseases 2021d: www.nih.go.jp/niid/ja/id/693-disease-based/ta/dengue/idsc/iasr-news/5754-pr4252.html). From the serological dengue data obtained from 207 individuals, ten individuals were seropositive for DENV without any travel history and five cases were completely asymptomatic. The comparison between seropositive and seronegative individuals revealed that seropositive individuals were significantly associated with increased length of stay in the park, but not with the individual mosquito prevention efforts. Further seroprevalence studies in and around cases would enable us to understand whether Yoyogi Park was an exception or whether there has been extensive silent DENV transmission.
The added utility of using a threshold approach to detecting aberrant incidence rates for public health activities remains to be developed, but could at least provide a basis for performing seroprevalence studies in and around cases detected during such high incidence weeks. Likewise, the choice of surveillance scale needs to be addressed. Greater Tokyo was sensitive to detecting outliers, but which may be a consequence of aggregating lower incidence prefectures with Tokyo. Insofar as public health decisions are taken at the prefecture level, establishing threshold levels at this scale may be more practical and appropriate.
In conclusion, while increased human movement and permissive temperatures do pose a threat for DENV invasion, evidence suggests that thus far Yoyogi was an exception and that a considerable viral biomass following repeated introduction may be required for successful viral implantation. Seroprevalence studies targeting areas with apparently unusual dengue incidences would go a long way in establishing the extent of the problem, at least for the present.
References
Alves MJ, Fernandes PL, Amaro F, et al (2013) Clinical presentation and laboratory findings for the first autochthonous cases of dengue fever in Madeira island, Portugal, October 2012. Euro Surveill 18:ii 20398
Bhatt S, Gething P, Brady O et al (2013) The global distribution and burden of dengue. Nature 496:504–507. https://doi.org/10.1038/nature12060
Chan M, Johansson MA (2012) The incubation periods of dengue viruses. PLoS ONE 7(11), e50972
Chen LH, Wilson ME (2008) The role of the traveler in emerging infections and magnitude of travel. Med Clin N Am 92:1409–1432. https://doi.org/10.1016/j.mcna.2008.07.005
Duong V, Lambrechts L, Paul RE, et al (2015) Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A, pp ii: 201508114
Effler PV, Pang L, Kitsutani P et al (2005) Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis 11:742–749
GenStat for Windows 15th Edition. Hemel Hempstead, UK: VSN International Ltd.
Geospatial Information Authority of Japan (2021) Map of geospatial information authority of Japan. http://maps.gsi.go.jp/. Accessed May 3 2021
Grange L, Simon-Loriere E, Sakuntabhai A, et al (2014) Epidemiological risk factors associated with high global frequency of inapparent dengue virus infections. Front Immunol, 5:280. 10.3389
Gubler D (2002) Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol 10:100–103
Gubler D, Kuno G (1997) Dengue and dengue hemorrhagic fever. CAB International, Wallingford
Hales S, de Wet N, Maindonald J et al (2002) Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet 360:830–834
Halstead SB (2007) Dengue. Lancet 370:1644–1652
JTB Tourism Research & Consulting Co. (2021) Japanese outbound tourists statistics. www.tourism.jp/en/tourism-database/stats/outbound/#annual. Accessed 3 May 2021
Kojima G (2015) Autochthonous dengue fever imported to England from Japan, 2014. Emerg Infect Dis 21:182–184. https://doi.org/10.3201/eid2101.141581
Kutsuna S, Kato Y, Moi ML et al (2014) (2015) Autochthonous dengue fever, Tokyo, Japan. Emerg Infect Dis 21(3):517–520. https://doi.org/10.3201/eid2103/141662
La Ruche G, Souarès Y, Armengaud A, et al (2010) First two autochthonous dengue virus infections in metropolitan France, Sept 2010. Euro Surveill 15:ii19676
Lambrechts L, Scott TW, Gubler DJ (2010) Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis4, e646. https://doi.org/10.1371/journal.pntd.0000646
Ministry of Health, Labour and Welfare (2011) Case definition of Dengue. www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou11/01-04-19.html. Accessed 3 May 2021
Ministry of Health, Labour and Welfare (2018) Infectious Disease Surveillance System in Japan. www.niid.go.jp/niid/images/epi/nesid/nesid_en.pdf. Accessed 3 May 3 2021
Misslin R, Telle O, Daudé E et al (2016) Urban climate versus global climate change—what makes the difference for dengue? Ann NY Acad Sci. https://doi.org/10.1111/nyas.13084
Murray KO, Rodriguez LF, Herrington E et al (2013) Identification of dengue fever cases in Houston, Texas, with evidence of autochthonous transmission between 2003 and 2005. Vector Borne Zoonotic Dis 13:835–845. https://doi.org/10.1089/vbz.2013.1413
Nakayama E, Kotaki A, Tajima S et al (2016) Two different dengue virus strains in the Japanese epidemics of 2014. Virus Genes 52(5):722–726. https://doi.org/10.1007/s11262-016-1356-4
National Institute of Infectious Diseases (2021) Annual report of Infectious Diseases Weekly Report. www.niid.go.jp/niid/ja/allarticles/surveillance/2270-idwr/nenpou/10115-idwr-nenpo2019.html. Accessed 3 May 2021
National Institute of Infectious Diseases (2021) Infectious Diseases Weekly Report. www.nih.go.jp/niid/ja/idwr.html. Accessed 3 May 2021
National Institute of Infectious Diseases (2021) Notification Trends Among Imported Dengue Cases in Japan. www.nih.go.jp/niid/ja/id/690-disease-based/ta/dengue/idsc/6663-dengue-imported.html. Accessed 3 May 2021
National Institute of Infectious Diseases (2021) Report on the results of active epidemiological investigation of dengue autochthonous infection cases, Infectious Diseases Weekly Report. www.nih.go.jp/niid/ja/id/693-disease-based/ta/dengue/idsc/iasr-news/5754-pr4252.html. Accessed 3 May 2021
Quam MB, Sessions O, Kamaraj US et al (2016) Dissecting Japan’s dengue outbreak in 2014. Am J Trop Med Hyg 94(2):409–412. https://doi.org/10.4269/ajtmh.15-0468
Reiter P, Gubler DJ (1997) Surveillance and control of urban dengue vectors. In: Gubler DJ, Kuno G (eds) Dengue and dengue hemorrhagic fever. CAB International, Oxford, pp 425–462
Schmidt-Chanasit J, Emmerich P, Tappe D, et al (2014) Autochthonous dengue virus infection in Japan imported into Germany, Sept 2013. Euro Surveill 19(3):ii 20681
Seki N, Iwashita Y, Moto R, et al (2015) An autochthonous outbreak of dengue type 1 in Tokyo, Japan 2014. Nihon Koshu Eisei Zasshi, 62(5):238–250. Japanese. https://doi.org/10.11236/jph.62.5_238
Senda A, Sakuntabhai A, Inaida S et al (2018) Estimating frequency of probable autochthonous cases of dengue Japan. Emerg Infect Dis 24(9):1705–1708. https://doi.org/10.3201/eid2409.170408
Statistics Bureau, Ministry of Internal Affairs and Communications (2017) The results of national census 2015. www.stat.go.jp/data/kokusei/2015/kekka.html. Accessed 3May 2021
Stoddard ST, Wearing HJ, Reiner RC Jr, et al (2014) Long-term and seasonal dynamics of dengue in Iquitos, Peru. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0003003
Sukehiro N, Kida N, Umezawa M, et al (2013) First report on invasion of yellow fever mosquito, Aedes aegypti, at Narita International Airport, Japan in August 2012. Japanese J Infect Dis 66(3):189–194. https://doi.org/10.7883/yoken.66.189
Taniguchi K, Yoshida M, Sunagawa T et al (2008) Imported infectious diseases and surveillance in Japan. Travel Med Infect Dis 6(6):349–354. https://doi.org/10.1016/j.tmaid.2008.07.001
Teissier Y, Paul R, Aubry M, et al (2020) Long-term persistence of monotypic dengue transmission in small size isolated populations, French Polynesia, 1978–2014, PLoS Negl Trop Dis.6 14(3):e0008110. https://doi.org/10.1371/journal.pntd.0008110
Thai KT, Anders KL (2011) The role of climate variability and change in the transmission dynamics and geographic distribution of dengue. Exp Biol Med 236944–54. https://doi.org/10.1258/ebm.2011.010402
Tuberculosis and Infectious Disease Control Division, Health Service Bureau, Ministry of Health, Labour and Welfare (2016) Clinical guideline of mosquito-borne diseases. http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000112494.pdf. Accessed 3 May 2021
WHO (2015) Dengue and dengue hemorrhagic fever, fact sheet 117.World Health Organization. http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed 5 May 2021
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 RIETI
About this chapter
Cite this chapter
Senda, A., Sakuntabhai, A., Matsuda, F., Paul, R. (2022). Potential Transmission of Dengue Virus in Japan. In: Yano, M., Matsuda, F., Sakuntabhai, A., Hirota, S. (eds) Socio-Life Science and the COVID-19 Outbreak. Economics, Law, and Institutions in Asia Pacific. Springer, Singapore. https://doi.org/10.1007/978-981-16-5727-6_11
Download citation
DOI: https://doi.org/10.1007/978-981-16-5727-6_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5726-9
Online ISBN: 978-981-16-5727-6
eBook Packages: Political Science and International StudiesPolitical Science and International Studies (R0)