Abstract
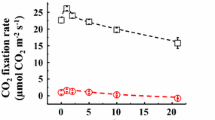
For maximal rates of CO2 assimilation in isolated intact spinach chloroplasts the generation of the adequate NADPH/ATP ratio is achieved either by cyclic electron flow around photosystem I or by linear electron transport to oxaloacetate, nitrite or oxygen (Mehler-reaction). The interrelationships between these poising mechanisms turn out to be strictly hierarchical. In the presence of antimycin A, an inhibitor of ferredoxin-dependent cyclic electron transport, the reduction of both, oxaloacetate and nitrite, but not that of oxygen restores CO2 fixation. When oxaloacetate and nitrite are added at low concentrations simultaneously during steady-state CO2 fixation, the reduction of nitrite is clearly preferred over the reduction of oxaloacetate, but CO2 fixation is not influenced. Nitrite reduction is not decreased upon addition of oxaloacetate, but vice versa. This is due to the regulation of NADP-malate dehydrogenase activation by electron pressure via the ferredoxin/thioredoxin system on the one hand, and by the NADPH/(NADP+NADPH) ratio (anabolic reduction charge, ARC) on the other hand. Thus the closing of the ‘malate valve’ prevents drainage of reducing equivalents from the chloroplast (1) when a low ARC indicates a high demand for NADPH in the stroma and (2) when nitrite reduction reduces the electron pressure at ferredoxin. The ‘malate valve’ is opened when cyclic electron transport is inhibited by antimycin A. Under these conditions the rate of malate formation is higher than in the absence of the inhibitor even in the presence of oxaloacetate, thus indicating that the regulation of the ‘malate valve’ functions at various redox states of the acceptor side of Photosystem I.
Similar content being viewed by others
Abbreviations
- ARC:
-
anabolic reduction charge (NADPH/(NADP+NADPH))
- Chl:
-
chlorophyll
- DTT:
-
dithiothreitol; Fd-ferredoxin
- NADP-MDH:
-
NADP-malate dehydrogenase
- OAA:
-
oxaloacetate
- PS:
-
photosystem
- qN:
-
non-photochemical quenching
- qP:
-
photochemical quenching
- ФE :
-
quantum efficiency of PS II
References
Arnon DI (1969) Role of ferredoxin in photosynthesis. Naturwissenschaften 56: 295–305
Baalmann E, Backhausen JE, Kitzmann C and Scheibe R (1994) Regulation of NADP-dependent glyceraldehyde 3-phosphate dehydrogenase activity in spinach chloroplasts. Bot Acta 107: in press
Drechler Z, Nelson N and Neuman J (1969) Antimycin A as an uncoupler and electron transport inhibitor in photoreactions of chloroplasts. Biochim Biophys Acta 189: 65–73
Foyer CH, Lelandais M and Harbinson J (1992) Control of the quantum efficiencies of photosystem I and II, electron flow, and enzyme activation following dark-to-light transitions in pea leaves. Plant Physiol 99: 979–986
Furbank RT and Horton P (1987) Regulation of photosynthesis in isolated barley protoplasts: the contribution of cyclic photophosphorylation. Biochim Biophys Acta 894: 332–338
Genty B, Briantais JM and Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92
Harbinson J, and Foyer C (1990) The relationship between CO2 assimilation and electron transport in leaves. Photosynth Res 25: 213–224
Hatch MD, Dröscher L, Flügge UI and Heldt HW (1984) A specific translocator for oxaloacetate transport in chloroplasts. FEBS Lett 178: 15–19
Heber U (1984) Flexibility of chloroplast metabolism. In: Sybesma C, (ed) Advances in Photosynthesis Research, Vol III, pp. 381–389, The Hague: Martinus Nijhoff/Dr W Junk Publishers
Heber U, Egneus H, Hanck U, Jensen M and Köster S (1978) Regulation of photosynthetic electron transport and photophosphorylation in intact chloroplasts and leaves of Spinacia oleracea L. Planta 143: 41–49
Heber U and Walker D (1992) Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol 100: 1621–1626
Heineke D, Riens B, Grosse H, Hoferichter P, Peter U, Flügge UI and Heldt HW (1991) Redox transfer across the inner chloroplast envelope membrane. Plant Physiol 95: 1131–1137
Heldt HW (1969) Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett 5: 11–14
Heldt HW (1980) Measurement of metabolite movement across the envelope and of the pH in the stroma and the thylakoid space of intact chloroplasts. Methods Enzymol 69: 604–613
Katona E, Neimanis S, Schönknecht G and Heber U (1992) Photosystem I-dependent cyclic electron transport is important in controlling photosystem II activity in leaves under conditions of water streess. Photosynth Res 34: 449–464
Krömer S, Stitt M and Heldt HW (1988) Mitochondrial oxidative phosphorylation participating in photosynthetic metabolism of a leaf cell. FEBS Lett 226: 352–356
Lance C and Rustin P (1984) The central role of malate in plant metabolism. Physiol Vég 22: 625–641
Lehner K and Heldt HW (1978) Dicarboxylate transport across the inner membrane of the chloroplast envelope. Biochim Biophys Acta 501: 531–544
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140: 315–322
Marsho TV, Behrens PW and Radmer RJ (1979) Photosynthetic oxygen reduction in isolated intact chloroplasts and cells from spinach. Plant Physiol 64: 656–659
Mills JD, Slovacek RE and Hind G (1978) Cyclic electron transport in isolated intact chloroplasts. Further studies with antimycin. Biochim Biophys Acta 504: 298–309
Moss DA and Bendall DS (1984) Cyclic electron transport in chloroplasts. The Q-cycle and the site of action of antimycin. Biochim Biophys Acta 767: 389–395
Nicholas DJD and Nason A (1957) Determination of nitrate and nitrite. Methods Enzymol 3: 981–986
Ort DR (1986) Energy transduction in oxygenic photosynthesis: An overview of structure and mechanism. In: Staehelin LA and Arntzen CJ, (eds) Encyclopedia of Plant Physiology, New Series, Vol 19: Photosynthesis III, pp. 143–196. Heidelberg, Springer.
Rathnam CKM (1978) Malate and dihydroxyacetone phosphate-dependent nitrate reduction in spinach leaf protoplasts. Plant Physiol 62: 220–223
Rich PR (1988) A critical examination of the supposed variable proton stoichiometry of the chloroplast cytochrome bf complex. Biochim Biophys Acta 932: 33–42
Robinson JM (1986) Carbon dioxide and nitrite photo-assimilatory processes do not intercompete for reducing equivalents in spinach and soybean leaf chloroplasts. Plant Physiol 80: 676–684
Robinson JM (1988) Spinach leaf chloroplast CO2 and NO2 - photoassimilations do not compete for photogenerated reductant. Manipulation of reductant levels by quantum flux density titrations. Plant Physiol 88: 1373–1380
Scheibe R (1987) NADP+-malate dehydrogenase in C3-plants: Regulation and role of a light-activated enzyme. Physiol Plant 71: 393–400
Scheibe R and Jacquot J-P (1983) NADP regulates the light activation of NADP-dependent malate dehydrogenase. Planta 157: 548–553
Scheibe R and Stitt M (1988) Comparison of NADP-malate dehydrogenase activation, Q A reduction and O2 evolution in spinach leaves. Plant Physiol Biochem 26: 473–481
Schreiber U, Schliwa U and Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62
Schreiber U and Neubauer C (1990) O2-dependent electron flor, membrane energization and the mechanism of non-photochemical quenching of chlorophyll fluorescence. Photosynth Res 25: 279–293
Slater TF and Sawyer B (1962) A colorimetric method for estimating the pyridine nucleotide content of small amounts of animal tissue. Nature 193: 454–456
Slovacek RE, Crowther D and Hind G (1980) Relative activities of linear and cyclic electron flows during chloroplast CO2 fixation. Biochim Biophys Acta 592: 495–505
Steiger HM and Beck E (1981) Formation of hydrogen peroxide and oxygen dependence of photosynthetic CO2 assimilation by intact chloroplasts. Plant Cell Physiol 22: 561–576
Usuda H (1988) Adenine nucleotide levels, the redox state of the NADP system, and assimilatory force in nonaqueously purified mesophyll chloroplasts from maize leaves under different light intensities. Plant Physiol 88: 1461–1468
Walker D (1987) The Use of the Oxygen Electrode and Fluorescence Probes in Simple Measurements of Photosynthesis Sheffield: Oxygraphics Limited, pp 117–120
Woo KC (1983) Evidence for cyclic photophosphorylation during 14CO2 fixation in intact chloroplasts. Plant Physiol 72: 313–320
Woo KC, Gerbaud A and Furbank RT (1983) Evidence for endogenous cyclic photophosphorylation in intact chloroplasts. 14CO2 fixation with dihydroxyacetone phosphate. Plant Physiol 71: 321–325
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. Hans Walter Heldt on the occasion of his 60th birthday.
Rights and permissions
About this article
Cite this article
Backhausen, J.E., Kitzmann, C. & Scheibe, R. Competition between electron acceptors in photosynthesis: Regulation of the malate valve during CO2 fixation and nitrite reduction. Photosynth Res 42, 75–86 (1994). https://doi.org/10.1007/BF00019060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019060