Abstract
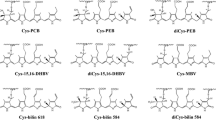
Each cryptomonad strain contains only a single spectroscopic type of biliprotein. These biliproteins are isolated as ≈50000 kDa αα'β2 complexes which carry one bilin on the α and three on the β subunit. Six different bilins are present on the cryptomonad biliproteins, two of which (phycocyanobilin and phycoerythrobilin) also occur in cyanobacterial and rhodophytan biliproteins, while four are known only in the cryptomonads. The β subunit is encoded on the chloroplast genome, whereas the α subunits are encoded by a small nuclear multigene family. The β subunits of all cryptomonad biliproteins, regardless of spectroscopic type, have highly conserved amino acid sequences, which show > 80% identity with those of rhodophytan phycoerythrin β subunits. In contrast, cyanobacteria and red algal chloroplasts each contain several spectroscopically distinct biliproteins organized into macromolecular complexes (phycobilisomes). The data on biliproteins, as well as several other lines of evidence, indicate that the cryptomonad biliprotein antenna system is ‘primitive’ and antedates that of the cyanobacteria. It is proposed that the gene encoding the cryptomonad biliprotein β subunit is the ancestral gene of the gene family encoding cyanobacterial and rhodophytan biliprotein α and β subunits.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- CER:
-
chloroplast endoplasmic reticulum
- SSU rRNA:
-
small subunit ribosomal RNA
References
Anderson LK and Grossman AR (1990) Structure and light-regulated expression of phycoerythrin genes in wild-type and phycobilisome assembly mutants of Synechocystis sp, strain PCC 7601. J Bacteriol 172: 1297–1305
Apt KE, Collier JL and Grossman AR (1995) Evolution of the phycobiliproteins. J Mol Biol 248: 79–86
Beale SI (1993) Biosynthesis of the phycobilins. Chem Rev 93: 785–802
Beale SI and Cornejo J (1991) Biosynthesis of the phycobilins-15,16-dihydrobiliverdin-IX-α is a partially reduced intermediate in the formation of phycobilins from biliverdin-IX-α. J Biol Chem 266: 22341–22345
Bryant DA (1982) Phycoerythrocyanin and phycoerythrin: Properties and occurrence in cyanobacteria. J Gen Microbiol 128: 835–844
Bryant DA (1992) Puzzles of chloroplast ancestry. Curr Biol 2: 240–242
Burger-Wiersma T, Veenhuis M, Korthals HJ, Van deWiel CCM and Mur LR (1986) A new prokaryote containing chlorophylls a and b. Nature 320: 262–264
Capuano V, Braux AS, DeMarsac NT and Houmard J (1991) The anchor polypeptide of cyanobacterial phycobilisomes — a molecular characterization of the Synechococcus sp. PCC 6301 apcE gene. J Biol Chem 266: 7239–7247
Cavalier-Smith T (1987) The simultaneous symbiotic origin of mitochondria, chloroplasts, and microbodies. Ann NY Acad Sci 53: 55–71
Cavalier-Smith T (1992) The number of symbiotic origins of organelles. BioSystems 28: 91–106
Chisholm SW, Frankel SL, Goericke R, Olson RJ, Palenik B, Waterbury JB, West-Johnsrud L and Zettler ER (1992) Prochlorococcus marinus nov. gen. nov. sp.: An oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b. Arch Microbiol 157: 297–300
deLorimier R, Chen CJ and Glazer AN (1992) Sequence comparison of two highly homologous phycoerythrins differing in bilin composition. Plant Mol Biol 20: 353–356
Dodge JD (1969) The ultrastructure of Chroomonas mesostigmatica Cutcher (Cryptophyceae). Arch Mikrobiol 69: 266–280
Dodge JD (1973) The Fine Structure of Algal Cells, p 86. Academic Press, London
Douglas SE (1992) Eukaryote-eukaryote endosymbioses: Insights from studies of a cryptomonad alga. BioSystems 28: 57–68
Douglas SE, Murphy CA, Spencer DF and Gray MW (1991) Cryptomonad algae are evolutionary chimaeras of two phylogenetically distinct unicellular eukaryotes. Nature 130: 148–151
Dubbs JM and Bryant DA (1991) Molecular cloning and transcriptional analysis of the cpeBA operon of the cyanobacterium Pseudanabaena species PCC 7409. Mol MIcrobiol 5: 3073–3085
Ducret A, Sidler W, Frank G and Zuber H (1994) The complete amino acid sequence of R-phycocyanin-I α and β subunits from the red alga Porphyridium cruentum. Structural and phylogenetic relationships of the phycocyanins within the phycobiliprotein families. Eur J Biochem 221: 563–580
Eschbach S, Wolters J and Sitte P (1991a) Primary and secondary structure of the nuclear small subunit ribosomal RNA of the cryptomonad Pyrenomonas salina as inferred from the gene sequence: Evolutionary implications. J Mol Evol 32: 247–252
Eschbach S, Hofmann CJB, Maier U-G, Sitte P and Hansmann P (1991b) A eukaryotic genome of 660 kb: karyotype of nucleomorph and cell nucleus of the cryptomonad alga, Pyrenomonas salina. Nucleic Acids Res 19: 1779–1781
Faust MA and Gantt E (1986) Effect of light intensity and glycerol on the growth, pigment composition, and ultrastructure of Chroomonas sp. J Phycol 6: 489–495
Gantt E (1979) Phycobiliproteins of cryptophyceae. In: Levandowsky M and Hutner SH (eds) Biochemistry and Physiology of Protozoa, Vol I, 2nd ed, pp 121–137. Academic Press, New York
Gantt E (1980) Structure and function of phycobilisomes: Light harvesting pigment complexes in red and blue-green algae. Int Rev Cytol 66: 45–80
Gantt E, Edwards MR and Provasoli L (1971) Chloroplast structure of the Cryptophyceae. Evidence for phycobiliproteins within intrathylakoidal spaces. J Cell Biol 48: 280–290
Gibbons A (1995) When it comes to evolution, humans are in the slow class. Science 267: 1907–1908
Gibbs S (1981a) The chloroplast endoplasmic reticulum: structure, function, and evolutionary significance. Int Rev Cytol 72: 49–99
Gibbs S (1981b) The chloroplasts of some algal groups may have evolved from endosymbiotic eukaryotic algae. Ann NY Acad Sci 361: 193–208
Gillott MA and Gibbs SP (1980) The cryptomonad nucleomorph: its ultrastructure and evolutionary significance. J Phycol 16: 558–568
Glazer AN (1976) Phycocyanins: structure and function. Photochem Photobiol Rev 1: 71–115
Glazer AN (1981) Photosynthetic accessory proteins with bilin prosthetic groups. In: Hatch MD Boardman NK (eds). The Biochemistry of Plants, Photosynthesis, Vol 8, pp 51–96. Academic Press, New York
Glazer AN (1983) Comparative biochemistry of photosynthetic light-harvesting systems. Ann Rev Biochem 52: 125–157
Glazer AN (1985) Light harvesting by phycobilisomes. Ann Rev Biophys Biophys Chem 14: 47–77
Glazer AN (1989) Light guides. Directional energy transfer in a photosynthetic antenna. J Biol Chem 264: 1–4
Glazer AN and Clark JH (1986) Phycobilisomes. Macromolecular structure and energy flow dynamics. Biophys J 49: 115–116
Glazer AN and Cohen-Bazire G (1975) A comparison of cryptophytan phycocyanins. Arch Microbiol 104: 29–32
Glazer AN, Apell GS, Hixson CS, Bryant DA, Rimon S and Brown DM (1976) Biliproteins of cyanobacteria and Rhodophyta: Homologous family of photosynthetic accessory pigments. Proc Natl Acad Sci USA 73: 428–431
Glazer AN, Lundell DJ, Yamanaka G and Williams RC (1983) The structure of a ‘simple’ phycobilisome. Ann Microbiol (Inst Pasteur) 134B: 159–180
Greenwood AD, Griffiths HB and Santore UJ (1977) Chloroplasts and cell compartments in Cryptophyceae. Br Phycol J 12: 119
Guard-Friar D and MacColl R (1986) Subunit separation (αα\s',β) of cryptomonad biliproteins. Photochem Photobiol 43: 81–85
Hayes JM (1983) Geochemical evidence bearing on the origin of aerobiosis, a speculative hypothesis. In: Schopf JW (ed), Earth's Earliest Biosphere. Its Origin and Evolution, pp 291–301. Princeton University Press, Princeton
Hibberd DJ and Norris RE (1984) Cytology and ultrastructure of Chlorarachnion reptans (Chlorarachniophyta diviso nova, Chloarachniophyceae classis nova). J Phycol 20: 385–394
Hiller RG and Martin CD (1987) Multiple forms of a type I phycoerythrin from a Chroomonas sp. (Cryptophyceae) varying in subunit composition. Biochim Biophys Acta 923: 98–102
Ingram K and Hiller RG (1983) Isolation and characterization of a major chlorophyll a/c 2 light-harvesting protein from a Chroomonas species (Cryptophyceae). Biochim Biophys Acta 772: 310–319
Jenkins J, Hiller RG, Speirs J and Godovac-Zimmermann J (1990) A genomic clone encoding a cryptophyte phycoerythrin α subunit. FEBS Lett 273: 191–194
Johnson PW and Sieburth JM (1979) Chroococcoid cyanobacteria: A ubiquitous and diverse photrophic biomass. Limnol Oceanogr 24: 928–935
Klotz AV and Glazer AN (1987) γ-N-Methylasparagine in phycobiliproteins. Occurrence, location, and biosynthesis. J Biol Chem 262: 17350–17355
Knoll AH (1994) Proterozoic and early Cambrian protists: Evidence for accelerating evolutionary tempo. Proc Natl Acad Sci USA 91: 6743–6750
Lewin RA and Cheng L (eds) (1989) Prochloron: A Microbial Enigma. Chapman and Hall, London
Li N and Cattolico RA (1987) Chloroplast genome characterization in the red alga Griffithsia pacifica. Mol Gen Genet 209: 343–351
Lichtlé C (1979) Effects of nitrogen deficiency and light intensity on Cryptomonas rufescens (Cryptophyceae). I. Cell and photosynthetic apparatus and encystment. Protoplasma 101: 283–293
Lichtlé C, Duval JC and Lemoine Y (1987) Comparative biochemical, functional and ultrastructural studies of photosystem particles from a Cryptophycea: Cryptomonas rufescens; isolation of an active phycoerythrin particle. Biochim Biophys Acta 894: 76–90
Lichtlé C, McKay RML and Gibbs SP (1992) Immunogold localization of Photosystem I and Photosystem II light-harvesting complexes in cryptomonad thylakoids. Biology Cell 74: 187–194
Ludwig M and Gibbs SP (1989) Localization of phycoerythrin at the lumenal surface of the thylakoid membrane in Rhodomonas lens. J Cell Biol 108: 875–884
Lundell DJ, Williams RC and Glazer AN (1981) Molecular architecture of a light-harvesting antenna. In vitro assembly of the rod substructures of Synechococcus 6301 phycobilisome. J Biol Chem 256: 3580–3592
MacColl R and Guard-Friar D (1987) Phycobiliproteins. Boca Raton. CRC Press Inc
Maerz M and Sitte P (1991) Isolation, physical map and gene map of mitochondrial DNA from the cryptomonad Pyrenomonas salina. Plant Mol Biol 16: 593–600
Maerz M, Wolters J, Hofmann CJB, Sitte P and Maier U-G (1992) Plastid DNA from Pyrenomonas salina (Cryptophyceae): Physical map, genes and evolutionary implications. Curr Genet 21: 73–81
Maier U-G (1992) The four genomes of the alga Pyrenomonas salina (Cryptophyta). BioSystems 28: 69–73
Maier U-G and Sitte P (1993/1994) Cryptomonad evolution: insights into a ‘eucyte within a eucyte’. Endocytobiosis and Cell Res 10: 129–135
Maier U-G, Hofmann CJB, Eschbach S, Wolters J and Igloi GL (1991) Demonstration of nucleomorph-encoded eukaryotic small subunit ribosomal RNA in cryptomonads. Mol Gen Genet 230: 155–160
Martin CD and Hiller RG (1987) Subunits and chromophores of a type I phycoerythrin from a Chroomonas sp. (Cryptophyceae) Biochim Biophys Acta 923: 88–97
Mazel D, Guglielmi G, Houmard J, Sidler W, Bryant DA and Tandeau de Marsac N (1986) Green light induces transcription of the phycoerythrin operon in the cyanobacterium Calothrix 7601. Nucleic Acids Res 14: 8279–8290
McFadden GI (1990) Evidence that cryptomonad chloroplasts evolved from photosynthetic eukaryotic endosymbionts. J Cell Sci 95: 303–308
McFadden GI, Gilson PR, Hofmann CJB, Adcock GJ and Maier U-G (1994) Evidence that an amoeba acquired a chloroplast by retaining part of an engulfed eukaryotic alga. Proc Natl Acad Sci USA 91: 3690–3694
Morden CW, Delwiche CF, Kuhsel M and Palmer JD (1992) Gene phylogenies and the endosymbiotic origin of plastids. BioSystems 28: 75–90
Mörschel E and Wehrmeyer W (1975) Multiple forms of phycoerythrin-545 from Cryptomonas maculata. Archiv Microbiol 113: 83–89
Mörschel E and Wehrmeyer W (1977) Cryptomonad biliprotein: Phycocyanin-645 from a Chroomonas species. Archiv Microbiol 105: 153–158
Ong LJ and Glazer AN (1991) Phycoerythrins of marine unicellular cyanobacteria. I. Bilin types and locations and energy transfer pathways in Synechococcus sp. phycoerythrins. J Biol Chem 266: 9515–9527
Palenik B and Haselkorn R (1992) Multiple evolutionary origins of prochlorophytes, the chlorophyll b-containing prokaryotes. Nature 355: 265–267
Palmer JD (1993) A genetic rainbow of plastids. Nature 364: 762–763
Raven PH (1970) A multiple origin for plastids and mitochondria. Science 169: 641–646
Reith M and Douglas SE (1990) Localization of β-phycoerythrin to the thylakoid lumen of Cryptomonas Ф does not require a signal peptide. Plant Mol Biol 15: 585–592
Rhiel E, Mörschel E and Wehrmeyer W (1985) Correlation of pigment deprivation and ultrastructural organization of thylakoid membranes in Cryptomonas maculata following nutrient deficiency. Protoplasma 129: 62–73
Rhiel E, Mörschel E and Wehrmeyer W (1987) Characterization and structural analysis of a chlorophyll a/c light-harvesting complex in Cryptomonas maculata (Cryptophyceae). Bot Acta 102: 46–53
Rhiel E, Kunz J and Wehrmeyer W (1989) Immunocytochemical localization of phycoerythrin-545 and of a chlorophyll a/c light harvesting complex in Cryptomonas maculata (Cryptophyceae). Bot Acta 102: 46–53
Roell MK and Morse DE (1993) Organization, expression and nucleotide sequence of the operon encoding the R-phycoerythrin α-subunit and β-subunit from the red alga Polysiphonia boldii. Plant Mol Biol 21: 47–58
Sidler W and Zuber H (1988) Structural and phylogenetic relationships of phycoerythrins from cyanobacteria, red algae and cryptophyceae. In: Scheer H and Schneider S (eds) Photosynthetic Light-Harvesting Systems, pp 49–60, Walter de Gruyter & Co., Berlin
Sidler W, Kumpf B, Suter F, Morisset W, Wehrmeyer W and Zuber H (1985) Structural studies on cryptomonad biliprotein subunits. Two different α-subunits in Chroomonas phycocyanin-645 and Cryptomonas phycoerythrin-545. Biol Chem Hoppe-Seyler 366: 233–244
Sidler W, Kumpf B, Suter F, Klotz AV, Glazer AN and Zuber H (1989) The complete amino-acid sequence of the α and β subunits of B-phycoerythrin from the rhodophytan alga Porphyridium cruentum. Biol Chem Hoppe-Seyler 370: 115–124
Sidler W, Nutt H, Kumpf B, Frank G, Suter F, Brenzel A, Wehrmeyer W and Zuber H (1990) The complete amino-acid sequence and the phylogenetic origin of phycocyanin-645 from the cryptophytan alga Chroomonas sp. Biol Chem Hoppe-Seyler 371: 537–547
Spear-Bernstein L and Miller KR (1985) Are the photosynthetic membranes of cryptophyte algae inside out? Protoplasma 129: 1–9
Spear-Bernstein L and Miller KR (1987) Immunogold localization of the phycobiliprotein of a cryptophyte alga to the intrathylakoidal space. In: Biggins J (ed) Progress in Photosynthesis Research, Vol 2, sect 4, pp 309–312. Martinus Nijhoff Publishers, Dordrecht
Swanson RV and Glazer AN (1990) Phycobiliprotein methylation. Effect of the γ-N-methylasparagine residue on energy transfer in phycocyanin and the phycobilisome. J Mol Biol 214: 787–796
Urbach E, Robertson DL and Chisholm SW (1992) Multiple evolutionary origins of prochlorophytes within the cyanobacterial radiation. Nature 355: 267–270
Waterbury JB, Watson SW, Guillard RRL and Brand LE (1979) widespread occurrence of a unicellular, marine, planctonic cyanobacterium. Nature 277: 293–294
Wedemayer GJ, Wemmer DE and Glazer AN (1991) Phycobilins of cryptophycean algae. Structures of novel bilins with acryloyl substituents from phycoerythrin 566. J Biol Chem 266: 4731–4741
Wedemayer GJ, Kidd DG, Wemmer DE and Glazer AN (1992) Phycobilins of cryptophycean algae. Occurrence of dihydrobiliverdin and mesobiliverdin in cryptomonad biliproteins. J Biol Chem 267: 7315–7331
Wehrmeyer W (1970) Zur Feinstruktur der chloroplasten einiger photoautotropher cryptophyceen. Arch Mikrobiol 71: 367–383
Wemmer DE, Wedemayer GJ and Glazer AN (1993) Phycobilins of cryptophycean algae. Novel linkage of dihydrobiliverdin in a phycoerythrin 555 and a phycocyanin 645. J Biol Chem 268: 1658–1669
Whatley JM and Whatley FR (1981) Chloroplast evolution. New Phytol 87: 233–247
Wilbanks SM and Glazer AN (1993) Phycoerythrins of marine unicellular cyanobacteria. III. Sequence of a class II phycoerythrin. J Biol Chem 266: 9535–9539
Wilbanks SM, Wedemayer GJ and Glazer AN (1989) Posttranslational modifications of the β subunit of a cryptomonad phycoerythrin. Sites of bilin attachment and asparagine methylation. J Biol Chem 264: 17860–17867
Zuber H (1983) Structure and function of the light-harvesting phycobiliproteins from the cyanobacterium Mastigocladus laminosus. In: Papageorgiou GC and Packer L (eds) Photosynthetic Prokaryotes: Cell Differentiation and Function, pp 23–42 Elsevier science Publishing Co, Inc, New York
Zuber H (1986) Structural principles and variability of light-harvesting antennae. In: Youvan DC and Daldal F (eds) Microbial Energy Transduction. Genetics, Structure, and Function of Membrane Proteins, pp 53–61. Current Communications in Molecular Biology, Cold Spring Harbor Laboratory, Cold Spring Harbor
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Glazer, A.N., Wedemayer, G.J. Cryptomonad biliproteins — an evolutionary perspective. Photosynth Res 46, 93–105 (1995). https://doi.org/10.1007/BF00020420
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020420