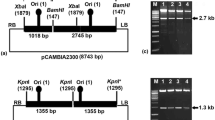
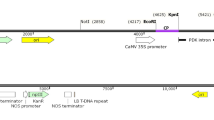
Summary
A hybrid Cauliflower Mosaic Virus (CaMV) genome containing a selectable marker gene was constructed by replacing the gene VI coding region with the aminoglycoside (neomycin) phosphotransferase type II [APH(3′)II] gene from Tn5. This modified viral genome was tested for its infectivity both in planta and in a protoplast transformation system of Brassica campestris var. rapa. Stable, genetically transformed cell lines of B. campestris var. rapa were obtained after transformation. DNA of the hybrid CaMV genome was found to be integrated into high molecular weight plant genomic DNA. Transformation was achieved only when the hybrid genome was supplied together with wild type viral DNA. A possible complementation of the modified CaMV genome with the wild type viral DNA as a helper molecule in planta and in the protoplast system is discussed.
Similar content being viewed by others
References
Bevan MW, Chilton M-D: T-DNA of the Agrobacterium Ti and Ri plasmids. Annual Rev Genet 16:357–384, 1982.
Bevan MW, Flavell RB, Chilton M-D: A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304:184–187, 1983.
DeBlock M, Herrera-Estrella L, VanMontagu M, Schell J, Zambyrski P: Expression of foreign genes in regenerated plants and their progeny. EMBO J 3:1681–1689, 1984.
Brisson N, Paszkowski J, Penswick JR, Gronenborn B, Potrykus I, Hohn T: Expression of a bacterial gene in plants by using a viral vector. Nature 310:511–514, 1984.
Broglie R, Coruzzi G, Fraley RT, Rogers SG, Horsch RB, Niedermeyer JG, Fink CL, Flick JS, Chua N-H: Light regulated expression of a pea ribulose-1-,5-bisphosphate carboxylase small subunit gene in transformed plant cells. Science 224:838–843, 1984.
Daubert S, Shephert RJ, Gardner RC: Insertional mutagenesis of the cauliflower mosaic virus genome. Gene 25:201–208, 1983.
Dixon LK, Hohn T: Cloning and manipulating cauliflower mosaic virus, in Recombinant DNA Research and Viruses. ed Y. Becker, in press, 1985.
Favali MA, Bassi M, Conti GG: A quantitative autoradiographic study of intracellular sites for replication of Cauliflower Mosaic Virus. Virology 53:115–119, 1973.
Fernandes SM, Lurquin PF, Kado CI: Incorporation and maintenance of recombinant-DNA plasmid vehicles pBR313 and pCR1 in plant protoplasts. FEBS Lett 87:277–282, 1978.
Fraley RT, Rogers SG, Horsch RB, Sanders PR, Flick JS, Adams SP, Bittner ML, Brand LA, Fink CL, Fry JS, Galluppi GR, Goldberg SB, Hoffmann NL, Woo SC: Expression of bacterial genes in plant cells. Proc Natl Acad Sci USA 80:4803–4807, 1983.
Gardner RC, Howarth AJ, Hahn P, Brown-Luedi M, Shephert RD, Messing J: The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acid Res 9:2871–2888, 1981.
Gheysen G, Dhaese P, Van Montague M, Shell J: DNA flux across genetic barriers: the crown gall phenomenon. In: Hohn B, Dennis E (eds) Plant Gene Research. Genetic flux in plants. pp 12–47, 1985.
Guilley H, Dudley RG, Jonard G, Balazs E, Richards KE: Transcription of Cauliflower Mosaic Virus DNA: Detection of promoter sequences, and characterization of transcripts. Cell 30:763–773, 1982.
Guilley H, Richards KE, Jonard G: Observations concerning the discontinuous DNA of Cauliflower Mosaic Virus. EMBO J 2:277–282, 1983.
Hain R, Stabel P, Czernilofsky AP, Steinbiss HH, Herrera-Estrella L, Schell J: Uptake, integration, expression and genetic transmission of a selectable chimeric gene by plant protoplasts. Mol Gen Genet 199:161–168, 1985.
Herrera-Estrella L, DeBlock M, Messens E, Hernalsteens J-P, VanMontagu M, Shell J: Chimeric genes as dominant selectable markers in plant cells. EMBO J 2:987–995, 1983.
Herrera-Estrella L, Depiker A, VanMontagu M, Shell J: Expression of chimeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 303:209–213, 1983.
Horsch RB, Fraley RT, Rogers SG, Sanders PR, Loyd A, Hoffmann N: Inheritance of functional foreign genes in plants. Science 223:496–498, 1984.
Howell SH, Walker LL, Dudley RK: Cloned cauliflower mosaic virus DNA infects turnips (Brassica rapa). Science 208:1265–1267, 1980.
Hull R, Covey SN: Does cauliflower mosaic virus replicate by reverse transcription. TIBS 8:119–121, 1983.
Jimenez A, Davies J: Expression of a transposable antibiotic resistance element in Saccharomyces. Nature 287:869–871, 1980.
Krens FA, Molendijk L, Wullems GJ, Schilperoort RA: In vitro transformation of plant protoplast with Ti-plasmid DNA. Nature 296:72–74, 1982.
Krens FA, Schilperoort RA: Ti-plasmid DNA uptake and expression by protoplasts of Nicotiana tabacum. In: Vasil IK (ed) Cell Culture and Somatic Cell Genetics of Plants, Vol. 1, Laboratory Techniques. Academic Press, New York, 1984, pp 522–534.
Lebeurier G, Hirth L, Hohn T, Hohn B: Infectivities of native and cloned DNA of cauliflower mosaic virus. Gene 12:139–146, 1980.
Lebeurier G, Hirth L, Hohn B, Hohn T: In vivo recombination of cauliflower mosaic virus DNA. Proc Natl Acad Sci USA 75:1428–1432, 1982.
Liang-cai L, Kohlenbach W: Somatic embryogenesis in quite a direct way in cultures of mesophyll protoplasts of Brassica napus. Plant Cell Rep 1:209–211, 1982.
Lorz H, Baker B, Schell J: Gene transfer to cereal cells mediated by protoplast transformation. Mol Gen Genet 199:178–182, 1985.
Odell JT, Nagy F, Chua N-H: Identification of DNA sequences required for activity of the cauliflower mosaic virus 35 S promoter. Nature 313:810–812, 1985.
Maniatis, Fritsch EF, Sambrook J: Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, New York, 1982.
Murai N, Sutton DW, Murray MG, Slighton JL, Merlo DJ, Reichert NA, Senupta-Goplan C, Stock CA, Barker RF, Kemp JD, Hall TC: Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science 222:476–482, 1983.
Paszkowski J, Shillito RD, Saul M, Mandak V, Hohn T, Hohn B, Potrykus I: Direct gene transfer to plants. EMBO J 3:2717–2722, 1984.
Paszkowski J, Shinshi H, Konig I, Lazar GB, Hohn T, Mandak V, Potrykus I: Proliferation of Cauliflower Mosaic Virus in protoplast-derived clones of turnip (Brassica rapa). In: Potrykus I et al. (eds) protoplasts 1983, Poster Proceedings. Birkhauser, Basel, 1983, pp 138–139.
Pfeiffer P, Hohn T: Involvement of reverse transcription in the replication of Cauliflower Mosaic Virus: A detailed model and test of some aspects. Cell 33:781–789, 1983.
Pisan B, Potrykus I, Paszkowski J: Optimalization of turnip (Brassica rapa) protoplast culture for Cauliflower Mosaic Virus transformation. In: Potrytus I et al. (eds) Protoplast 1983, Poster Proceedings. Birkhauser, Basel, 1983, pp 44–45.
Potrykus I, Paszkowski J, Saul MW, Petruska J, Shillito RD: Molecular and general genetics of a hybrid foreign gene introduced to tobacco by direct gene transfer. Mol Gen Genet 199:169–177, 1985.
Potrykus I, Saul M, Petruska I, Paszkowski J, Shillito RD: Direct gene transfer to cells of a Graminaceous monocot. Mol Gen Genet 199:183–188, 1985.
Reiss B, Sprengel R, Will H, Schaller H: A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene 30:211–218, 1984.
Schocher RJ, Shillito RD, Saul MW, Paszkowski J, Potrykus I: Co-transformation of foreign genes to plants. submited.
Shillito RD, Paszkowski J, Potrykus I: Agarose plating and a bead type culture technique enable and stimulate development of protoplast-derived colonies in number of plant species. Plant Cell Rep 2:244–247, 1983.
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517, 1975.
Xiong C, Muller S, Lebeurier G, Hirth L: Identification by immunoprecipitation of cauliflower mosaic virus in vitro major translation product with a specific serum against viroplasm protein. EMBO J 1:971–976, 1982.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paszkowski, J., Pisan, B., Shillito, R.D. et al. Genetic transformation of Brassica campestris var. rapa protoplasts with an engineered cauliflower mosaic virus genome. Plant Mol Biol 6, 303–312 (1986). https://doi.org/10.1007/BF00034937
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00034937