Abstract
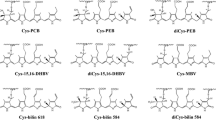
Two crytophycean phycocyanins (Cr-PCs), Hemiselmis strain HP9001 Cr-PC 612 and Falcomonas daucoides Cr-PC 69 were purified and characterized with respect to bilin numbers, types and locations. Each biliprotein carried one bilin on the α subunit and three on the β subunit. Cr-PC 612 carried phycocyanobilin at α-Cys-18, β-Cys-82, and β-Cys-158, and a doubly-linked 15,16-dihydrobiliverdin at β-DiCys-50,61. Cr-PC 569 carried phycocyanobilin at α-Cys-18 and β-Cys-82, a singly-linked Bilin 584 at β-Cys-158, and a doubly-linked Bilin 584 at β-DiCys-50,61. This work, in conjunction with earlier studies on Cr-PE 545, Cr-PE 555, Cr-PE 566, and Cr-PC 645, shows that there is no conserved location for the bilin with longest wavelength visible absorption band among these proteins, and, consequently, that there is no conserved energy transfer pathway common to all native cryptophycean biliproteins. Only phycocyanobilin or phycoerythrobilin is found at β-Cys-82; there is greater bilin variability at the other three attachment sites.
Similar content being viewed by others
Abbreviations
- Cr-PC:
-
cryptophycean phycocyanin
- Cr-PE:
-
cryptophycean phycoerythrin
- DBV:
-
15,16-dihydrobiliverdin
- MBV:
-
mesobiliverdin
- PCB:
-
phycocyanobilin
- PEB:
-
phycoerythrobilin
- HPLC:
-
high performance liquid chromatography
- TFA:
-
trifluoroacetic acid
References
Allen MB, Dougherty EC and McLaughlin JJA (1959) Chromoprotein pigments in flagellates. Nature 184: 1047–1049
Douglas SE (1992) Eukaryote-eukaryote endosymbioses: Insights from studies of a cryptomonad alga. BioSystems 28: 57–68
Ficner R, Lobeck K, Schmidt G and Huber R (1992) Isolation, crystallization, crystal structure analysis and refinement of B-phycoerythrin from the red alga Porphyridium sordidum at 2.2 Å resolution. J Mol Biol 228: 935–950
Glazer AN (1984) Phycobilisome. A macromolecular complex optimized for energy transfer. Biochim Biophys Acta 768: 29–51
Glazer AN and Apell GS (1977) A common evolutionary origin for the biliproteins of cyanobacteria, rhodophyta and cryptophyta. FEMS Lett 1: 113–116
Glazer AN and Fang S (1973) Chromophore content of blue-green algal phycobiliproteins. J Biol Chem 248: 659–662
Glazer AN and Wedemayer GJ (1995) Cryptomonad biliproteinsan evolutionary perspective. Photosynth Res 46: 93–105
Guard-Friar D and MacColl R (1986) Subunit separation (α, α′, β) of cryptomonad biliproteins. Photochem Photobiol 43: 81–85
Haxo FT and Fork DC (1959) Photosynthetically active accessory pigments of cryptomonads. Nature 184: 1051–1052
Hill DRA and Rowan KS (1989) The biliproteins of the Cryptophyceae. Phycologia 28: 455–463
Jenkins J, Hiller RG, Speirs J and Godovac-Zimmermann J (1990) A genomic clone encoding a cryptophyte α-subunit. Evidence for three α-subunits and an N-terminal membrane transit sequence. FEBS Lett 273: 191–194
Keller MD, Selvin RC, Claus W and Guillard RRL (1987) Media for the culture of oceanic ultraphytoplankton. J Phycol 23: 633–638
Mörschel E and Wehrmeyer W (1975) Cryptomonad biliprotein: Phycocyanin 645 from a Chroomonas species. Arch Microbiol 105: 153–158
ÓhEocha C and Raftery M (1959) Phycoerythrins and phycocyanins of cryptomonads. Nature 184: 1049–1051
Ong LJ and Glazer AN (1987) R-Phycocyanin II, a new phycocyanin occurring in marine Synechococcus species. Identification of the terminal energy acceptor in phycocyanins. J Biol Chem 262: 6323–6327
Ong LJ and Glazer AN (1991) Phycoerythrins of marine unicellular cyanobacteria. I. Bilin types and locations and energy transfer pathways in Synechococcus spp. phycoerythrins. J Biol Chem 266: 9515–9527
Sidler W, Kumpf B, Suter F, Morisset W, Wehrmeyer W and Zuber H (1985) Structural studies on cryptomonad biliprotein subunits. Two different α subunits in Chroomonas phycocyanin-645 and Cryptomonas phycoerythrin-545. Biol Chem Hoppe-Seyler 366: 233–244
Sidler W, Nutt H, Kumpf B, Frank G, Suter F, Brenzel A, Wehrmeyer W and Zuber H (1990) The complete amino-acid sequence and the phylogenetic origin of phycocyanin-645 from the cryptophytan alga Chroomonas sp. Biol Chem Hoppe-Seyler 371: 537–547
Swanson RV and Glazer AN (1990) Separation of phycobiliproteim subunits by reverse-phase high-pressure liquid chromatography. Anal Biochem 188: 295–299
Wedemayer GJ, Wemmer DE and Glazer AN (1991) Phycobilins of cryptophycean algae. Structures of novel bilins with acryloyl substituents from phycoerythrin 566. J Biol Chem 266: 4731–4741
Wedemayer GJ, Kidd DG, Wemmer DE and Glazer AN (1992) Phycobilins of cryptophycean algae. Occurrence of dihydrobiliverdin and mesobiliverdin in cryptomonad biliproteins. J Biol Chem 267: 7315–7331
Wemmer DE, Wedemayer GJ and Glazer AN (1993) Phycobilins of cryptophycean algae. Novel linkage of dihydrobiliverdin in a phycoerythrin 555 and a phycocyanin 645. J Biol Chem 268: 1658–1669
Wilbanks S, Wedemayer GJ and Glazer AN (1989) Posttranslational modifications of the β subunit of a cryptomonad phycoerythrin. Sites of bilin attachment and asparagine methylation. J Biol Chem 264: 17860–17867
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wedemayer, G.J., Kidd, D.G. & Glazer, A.N. Cryptomonad biliproteins: Bilin types and locations. Photosynth Res 48, 163–170 (1996). https://doi.org/10.1007/BF00041006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00041006