Summary
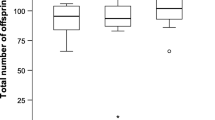
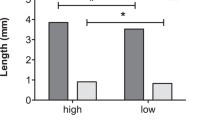
In many insects nutrients transferred by the male to the female at mating are later incorporated into both the eggs and soma of the mated females. Accordingly, it has been suggested that female insects can use these male-derived nutrients both for somatic maintenance and to increase both the number and quality of their offspring. Moreover, much discussion is presently devoted to whether the male nuptial gift represents paternal investment, defined as “any increase in given male's total surviving progeny by increasing the reproductive output by a given female”, or mating effort which obtains “if a male gains by increasing the proportion of eggs he fertilizes from a given female” (Parker and Simmons 1989). If the male nuptial gift represents parental investment it should be expected to benefit predominantly the offspring sired by the donor, whereas the “physiological fate” of the male nuptial gift is somewhat irrelevant under the mating effort explanation. In this paper we test these issues by studying the lifetime fecundity, egg weights and longevity of two groups of females of the polyandrous green-veined white butterfly, Pieris napi, one group of which was allowed to mate only once and the other of which was allowed to mate at liberty, the latter group of females mating on average 2.28 times. Moreover, to test the incorporation rate of male-derived nutrients, we performed a second set of experiments where females were allowed to mate with radioactively labelled males. The results showed that polyandrous females had higher lifetime fecundity compared to monandrous females, laying on average 1.61 as many eggs, and that the difference in cumulative fecundity between the two groups was statistically significant from the 5th day of egg-laying onwards. Polyandrous females also lived longer and maintained egg weight at a high level for longer than monandrous females. Largely concomitant with egg-laying rate, incorporation rate of male-derived nutrients peaked 3–4 days after mating, subsequently tapering off to stabilize at about 40% of the maximum. Given the opportunity, female P. napi remated after 3–5 days, the duration of the refractory period being positively correlated with ejaculate mass. Hence, although the nutrient investment of the first male to mate with a female “subsidizes” the progeny of later-mating males, the male nuptial gift in P. napi clearly qualifies as both paternal investment and mating effort.
Similar content being viewed by others
References
Baker HG, Baker I (1973) Amino acids in nectar and their evolutionary significance. Nature 241:543–545
Boggs CL (1981a) Selection pressures affecting male nutrient investment at mating in Heliconiine butterflies. Evolution 35:931–940
Boggs CL (1981b) Nutritional and life-history determinants of resource allocation in holometabolous insects. Am Nat 117:692–709
Boggs CL (1990) A general model of the role of male-donated nutrients in female insect's reproduction. Am Nat 136:598–617
Boggs CL, Gilbert LE (1979) Male contribution to egg production in butterflies evidence for transfer of nutrients at mating. Science 206:83–84
Boggs CL, Watt WB (1981) Population structure of pierid butterflies. IV. Genetic and physiological investment in offspring by male Colias. Occologica 50:320–324
Boggs CL, Smiley JT, Gilbert LE (1981) Patterns of pollen exploitation by Heliconius butterflies. Oecologia 48:284–289
Bowen BJ, Codd CG, Gwynne DT (1984) The katydid spermatophore (Orthoptera: Tettigoniidae): Male nutritional investment and its fate in the mated female. Aust J Zool. 32:23–31
Burns JM (1968) Mating frequency in natural populations of skippers and butterflies as determined by spermatophore counts. Proc Natl Acad Sci USA 61:852–859
Chen PS (1984) The functional morphology and biochemistry of insect male accessory glands and their secretions. Annu Rev Entomol 29:233–255
Drummond BA III (1984) Multiple mating and sperm competition in the Lepidoptera. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic, New York, pp 291–370
Ehrlich AH, Ehrlich PH (1978) Reproductive strategies in the butterflies: I. Mating frequency, plugging, and egg number. J Kansas Entomol Soc 51:666–697
Forsberg J, Wiklund C (1989) Mating in the afternoon: time saving in courtship and remating by females of a polyandrous butterfly, Pieris napi. Behav Ecol Sociobiol 25:349–356
Gilbert LE (1972) Pollen feeding and reproductive biology of Heliconius butterflies. Proc Natl Acad Sci USA 69:1403–1407
Gromko MH, Gilbert DG, Richmond RC (1984) Sperm transfer and use in the multiple mating system of Drosophila. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic, New York, pp 371–426
Gwynne DT (1984) Courtship feeding increased female reproductive success in bush crickets. Nature 307:361–363
Gwynne DT (1986) Stepfathers in insects and their pseudo-paternal investment. Ethology 71:74–77
Gwynne DT (1988) Courtship feeding benefits the mating male's offspring. Behav Ecol Sociobiol 23:373–377
Herman WS, Barker JF (1977) Effect of mating on monarch butterfly oogenesis. Experentia 33:688–689
Jones KN, Odendaal FJ, Ehrlich PR (1986) Evidence against the spermatophore as paternal investmant in the checkerspot butterflies (Euphydryas: Nymphalidae) Am Midl Nat 116:1–6
Labine P (1964) Population biology of the butterfly, Euphydryas editha. I. Barriers to multiple inseminations. Evolution 18:335–336
Labine P (1966) The population biology of the butterfly, Euphydryas editha. IV. Sperm precedence — a preliminary report. Evolution 20:580–586
Markow TA (1988) Drosophila males provide a material contribution to offspring sired by other males. Funct Ecol 2:77–79
Markow TA, Gallagher PD, Krebs RA (1990) Ejaculate-derived nutritional contribution and female reproductive success in Drosophila mojavensis. Funct Ecol 4:67–73
Obara Y (1982) Mate refusal hormone in the cabbage white butterfly? Naturwissenschaften 69:551–552
Obara Y, Tateda H, Kuwabara M (1975) Mating behaviour of the cabbage white butterfly, Pieris rapae crucivora. V. Copulatory stimuli inducing changes of female response patterns. Zool Mag 84:71–76
Oberhauser K (1988) Male monarch butterfly spermatophore mass and mating strategies. Anim Behav 36:1384–1388
Oberhauser K (1989) Effects of spermatophores on male and female monarch butterfly reproductive success. Behav Ecol Sociobiol 25:237–246
Oberhauser K (1992) Rate of ejaculate breakdown and intermating intervals in monarch butterflies. Behav Ecol Sociobiol 31:367–373
Parker G, Simmons L (1989) Nuptial feeding in insects: Theoretical models of male and female interests. Ethology 82:3–26
Pitnick S, Markow TA, Riedy MF (1991) Transfer of ejaculate and incorporation of male-derived substances by females in the nannoptera species group (Diptera: Drosophilidae). Evolution 45:774–780
Rothschild M (1978) Hell's angels. Antenna 2:38–39
Rutowski RL, Gilchrist GW (1986) Copulation in Colias eurytheme (Lepidoptera: Pieridae): patterns and frequency. J Zool 209:115–124
Rutowski RL, Gilchrist GW, Terkanian B (1987) Female butterflies mated with recently mated males show reduced reproductive output. Behav Ecol Sociobiol 20:319–322
Simmons L (1988) The contribution of multiple mating and spermatophore consumption to the lifetime reproductive success of female field crickets (Gryllus bimaculatus). Ecol Entomol 13:57–69
Simmons L, Parker G (1989) Nuptial feeding in insects: Mating effort versus paternal investment. Ethology 81:332–343
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, New York
Sugawara C (1979) Stretch reception in bursa copulatrix of the butterfly, Pieris rapae crucivora, and its role in behaviour. J Comp Physiol 130:191–199
Svärd L, Wiklund C (1986) Different ejaculate delivey strategies in first versus subsequent matings in the swallowtail butterfly Papilio machaon. Behav Ecol Sociobiol 18:325–330
Svärd L, Wiklund C (1998) Fecundity, egg weight and longevity in relation to multiple matings in the monarch butterfly. Behav Ecol Sociobiol 23:39–43
Svärd L, Wiklund C (1989) Mass and production rate of ejaculates in relation to monandry/polyandry in butterflies. Behav Ecol Sociobiol 24: 395–402
Svärd L, Wiklund C (1991) The effect of ejaculate mass on female reproductive output in the European swallowtail butterfly Papilio machaon. J Insect Behavior 4:33–41
Watanabe M (1988) Multiple matings increase the fecundity of the yellow swallowtail butterfly, Papilio xuthus, in summer generations. J Insect Behavior 1:17–30
Watt WB, Hoch PC, Mills SG (1974) Nectar resource use by Colias butterflies. Chemical and visual aspects. Oecologia 14:353–374
Wedell N, Arak A (1989) The wartbiter spermatophore and its effect on female reproductive output (Orthoptera: Tettigoniidae, Decticus verrucivorus). Behav Ecol Sociobiol 24:117–125
Wedell N (1992) Protandry, spermatophore size and mate assessment in the wartbiter Decticus verucivorus (Orthoptera: Tettigoniidae). Behav Ecol Sociobiol 31:301–308
Wickler W (1985) Stepfathers in insects and their pseudo-paternal investment. Z Tierpsychol 69:72–78
Wickler W (1986) Mating costs versus paternal investment: a reply to Gwynne. Ethology 71:78–79
Wiklund C, Forsberg J (1985) Courtship and mate discrimination between virgin and mated females in the orange tip butterfly, Anthocharis cardamines. Anim Behav 34:328–332
Wiklund C, Forsberg J (1991) Sexual size dimorphism in relation to female polygamy and protandry in butterflies: a comparative study of Swedish Pieridae and Satyridae. Oikos 60:373–381
Author information
Authors and Affiliations
Additional information
Correspondence to: C. Wiklund
Rights and permissions
About this article
Cite this article
Wiklund, C., Kaitala, A., Lindfors, V. et al. Polyandry and its effect on female reproduction in the green-veined white butterfly (Pieris napi L.). Behav Ecol Sociobiol 33, 25–33 (1993). https://doi.org/10.1007/BF00164343
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164343