Summary
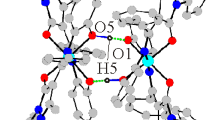
The following coordination compounds derived from 2-guanidinobenzimidazole (2GB) (1); [Ni(2GB)2]Cl2· H2O, (2); [Ni(2GB)2]Br2·3H2O, (3); [Ni(2GB)2-(NO3)2, (4); [Ni(2GB)2](OAc)2, (5); [Cu(2GB)Cl2], (6); [Cu(2GB)Br2], (7); [Cu(2GB)2]Br2·2H2O, (8); [Cu(2GB)2](NO3)2·H2O, (9); [Cu(2GB)2](OAc)2· H2O, (10); [Zn(2GB)Cl2]·H2O, (11); [Zn(2GB)Br2]·H2O, (12); [Co(2GB)Cl2(H2O)2]·5H2O, (13); [Co-(2GB)2Cl2]·3H2O, (14); [Co(2GB)2(H2O)2](NO3)2· 4H2O, (15); and [Co(2GB)2(H2O)2](OAc)2, (16) have been synthesized and characterized by i.r. and electronic spectroscopy. In addition (6)–(10) were analysed by e.p.r. The X-ray diffraction structure of compound (4) was obtained. It crystallizes in the monoclinic system, C2/c (a = 22.511(7), b = 6.735(6) and c= 15.345(5)Å, β =115.31(3)°, Z = 4, final R = 0.0360 and R w = 0.0388 for 1167 observed independent reflections). The nickel(II) atom coordinates two ligands in a square-planar geometry through the imidazolic N(3) and the guanidino N(12).
The probable ligand isomers involved in the coordination were determined by theoretical calculations, and the possible structures of the coordination compounds were investigated in order to verify that the experimentally proposed structures were stable. Two different types of coordination compounds were found. One, where the ligand is chelating through the imidazolic N(3) and the guanidino N(12), which is the case for most of the complexes [(2)–(13)]. With only one ligand in the coordination sphere, the structure was either tetrahedral (copper and zinc chloride and bromide complexes) or octahedral (cobalt). With two chelating 2GB units a square-planar geometry was stabilized [(2)–(5) and (8)–(10)]. The second type of coordination behaviour was observed in the cobalt compounds [(14)–(16)]. Here the ligand coordinates monodentate through the imidazolic N(3); the structure is tetrahedral.
Similar content being viewed by others
References
W. B. Pratt, Chemotherapy of Infection, Oxford University Press, New York, 1977.
N. Barba-Behrens, J. L. Bautista, M. E. Ruiz, P. Joseph-Nathan, A. Flores-Parra and R. Contreras, J. Inorg. Biochem., 40, 201 (1990).
H. Bhushan, L. K. Mishra and N. K. Jha, Ind. J. Chem., 21A, 1135 (1982).
P. D. Singh and L. K. Mishra, J. Ind. Chem. Soc., 58, 434 (1981).
A. K. Banerjee and S. P. Ghosh, J. Ind. Chem. Soc., 38, 237 (1961).
B. Lotina-Hennsen, S. Castillo-Acosta, N. Barba-Behrens, A. Vázquez-Olmos and S. E. Castillo-Blum, in the press.
N. Walker and D. Stuart, Acta Crystallogr., 39, 158 (1983).
D. J. Watkin, J. R. Carruthers and P. W. Betteridge, CRYSTALS, An Advanced Crystallographic Program System; Chemical Crystallography Laboratory, University of Oxford, Oxford, UK, 1988.
International Tables for X-ray Crystallography, Vol. IV, Kynoch Press, Birmingham, UK, 1974.
G. M. Sheldrick, SHELXS86, Program for Crystal Structure Solution, University of Göttingen, Göttingen, 1986.
PCMODEL, Version 4.0, Serena Software, Bloomington, IN, USA.
MOPAC, Version 6.0, Serena Software, Bloomington, IN, USA.
G. Höjer and S. Meza, Acta Chem. Scand., 26, 3723 (1972).
ICON8, QCPE No. 344, Quantum Chemistry Program Exchange, Chemistry Department, Indiana University, Bloomington, IN, USA. (The actual program used is a substantially modified version for PCs.)
B. J. Hathaway in G. Wilkinson (Ed.), Comprehensive Chemistry, Vol. 5, Pergamon Press, UK, 1987, Ch. 53.
B. A. Goodman and J. B. Raynor in A. G. Sharpe and H. J. Emeléus (Eds), Advances in Inorg. Chem. Radiochem., 135 (1970).
P. J. Steel, J. Heterocycl. Chem., 28, 1817 (1991).
E. Grundemann, H. Graubaum, D. Martin and E. Schiewald, Magn. Reson. Chem., 24, 21 (1986).
C. Acerete, J. Catalán, F. Febero, M. Sánchez-Cabezudo, R. M. Claramunt and J. Elguero, J. Heterocyclic Chem., 26, 1581 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barba-Behrens, N., Vázquez-Olmos, A., Castillo-Blum, S.E. et al. Coordination behaviour of 2-guanidinobenzimidazole towards cobalt(II), nickel(II), copper(II) and zinc(II). An experimental and theoretical study. Transition Met Chem 21, 31–37 (1996). https://doi.org/10.1007/BF00166009
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00166009