Summary
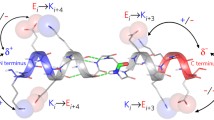
The contribution of peptide groups to Hα and Hβ proton chemical shifts can be modeled with empirical equations that represent magnetic anisotropy and electrostatic interactions [Ösapay, K. and Case, D.A. (1991) J. Am. Chem. Soc., 113, 9436–9444]. Using these, a model for the ‘random coil’ reference state can be generated by averaging a dipeptide over energetically allowed regions of torsion-angle space. Such calculations support the notion that the empirical constant used in earlier studies arises from neighboring peptide contributions in the reference state, and suggest that special values be used for glycine and proline residues, which differ significantly from other residues in their allowed ϕ,ψ-ranges. New constants for these residues are reported that provide significant improvements in predicted backbone shifts. To illustrate how secondary structure affects backbone chemical shifts we report calculations on oligopeptide models for helices, sheets and turns. In addition to suggesting a physical mechanism for the widely recognized average difference between α and β secondary structures, these models suggest several additional regularities that should be expected: (a) Hα protons at the edges of β-sheets will have a two-residue periodicity; (b) the Hα2 and Hα3 protons of glycine residues will exhibit different shifts, particularly in sheets; (c) Hβ protons will also be sensitive to local secondary structure, but in different directions and to a smaller extent than Hα protons; (d) Hα protons in turns will generally be shifted upfield, except those in position 3 of type I turns. Examples of observed shift patterns in several proteins illustrate the application of these ideas.
Similar content being viewed by others
References
Andersen N.H., Cao B. and Chen C. (1992) Biochem. Biophys. Res. Commun., 184, 1008–1014.
Anderson A.G. and Hermans J. (1988) Protein Struct. Funct. Genet., 3, 262–265.
Asakura T., Niizawa Y. and Williamson M.P. (1992) J. Magn. Reson., 98, 646–653.
Bashford D., Case D.A., Dalvit C., Tennant L. and Wright P.E. (1993) Biochemistry, 32, 8045–8056.
Bashford D. and Gerwert K. (1992) J. Mol. Biol., 224, 473–486.
Bashford D. and Karplus M. (1990) Biochemistry, 29, 10219–10225.
Blanco F.J., Herranz J., González C., Jiménez M.A., Rico M., Santoro J. and Nieto J.L. (1992) J. Am. Chem. Soc., 114, 9676–9677.
Bondi A. (1964) J. Chem. Phys. 40, 441–451.
Brooks B.R., Bruccoleri R.E., Olafson B.D., States D.J., Swaminathan S. and Karplus M. (1983) J. Comput. Chem., 4, 187–217.
BrooksIII C.L. and Case D.A. (1993) Chem. Rev., 93, 2487–2502.
Bruix M., Perello M., Herranz J., Rico M. and Nieto M.L. (1990) Biochem. Biophys. Res. Commun., 167, 1009–1014.
Buckingham A.D. (1960) Can. J. Chem., 38, 300–307.
Bundi A. and Wüthrich K. (1979) Biopolymers, 18, 285–297.
Chazin W.J. and Wright P.E. (1988) J. Mol. Biol., 202, 623–636.
Chen Y., Reizer J., SaierJr. M.H., Fairbrother W.J. and Wright P.E. (1993) Biochemistry, 32, 32–37.
Cross K.J. and Wright P.E. (1985) J. Magn. Reson., 64, 220–231.
Dalgarno D.C., Levine B.A. and Williams R.J.P. (1983) Biosci. Rep., 3, 443–452.
De Dios A.C., Pearson J.G. and Oldfield E. (1993) Science, 260, 1491–1496.
Distefano D.L. and Wand A.J. (1987) Biochemistry, 26, 7272–7281.
Finzel B.C., Clancy L.L., Holland D.R., Muchmore S.W., Watenpaugh K.D. and Einspahr H.M. (1989) J. Mol. Biol., 209, 779–791.
Grant J.A., Williams R.L. and Scheraga H.A. (1990) Biopolymers, 30, 929–949.
Guss J.M., Harrowell P.R., Murata M., Norris V.A. and Freeman H.C. (1986) J. Mol. Biol., 192, 361–387.
Haigh C.W. and Mallion R.B. (1980) Prog. NMR Spectrosc., 13, 303–344.
Harris R.K. (1986) Nuclear Magnetic Resonance Spectroscopy — A Physicochemical View, Longman, Harlow.
Honig B., Sharp K. and Yang A.-S. (1993) J. Phys. Chem., 97, 1101–1109.
Jiménez M.A., Blanco F.J., Rico M., Santoro J., Herranz J. and Nieto J.L. (1992) Eur. J. Biochem., 207, 39–49.
Kline A.D. and Wüthrich K. (1986) J. Mol. Biol., 192, 869–890.
Kuntz I.D., Kosen P.A. and Craig E.C. (1991) J. Am. Chem. Soc., 113, 1406–1408.
Lee M.S., PalmerIII A.G. and Wright P.E. (1992) J. Biomol. NMR, 2, 307–322.
McConnell H.M. (1957) J. Chem. Phys., 27, 226–229.
Moore J.M., Lepre C., Gippert G.P., Chazin W.J., Case D.A. and Wright P.E. (1991) J. Mol. Biol. 221, 533–555.
Ösapay K. and Case D.A. (1991) J. Am. Chem. Soc., 113, 9436–9444.
Ösapay K., Case D.A. and Cross K.J. (1991) SHIFTS Program, The Scripps Research Institute, La Jolla, CA.
Pardi A., Wagner G. and Wüthrich K. (1983) Eur. J. Biochem., 137, 445–454.
Pastore A. and Saudek V. (1990) J. Magn. Reson., 90, 165–176.
Pearlman D.A., Case D.A., Caldwell J.C., Seibel G.L., Singh U.C., Weiner P. and Kollman P.A. (1991) AMBER 4.0, University of California, San Francisco, CA.
Pettitt M. and Karplus M. (1988) J. Phys. Chem., 92, 3994–3997.
Pflugrath J.W., Wiegand G., Huber R. and Vertesy L. (1986) J. Mol. Biol., 189, 383–386.
Priestle J.P., Shaer H.-P. and Gruetter M.G. (1989) Proc. Natl. Acad. Sci. USA, 86, 9667–9671.
Richardson J.S. (1981) Adv. Protein Chem., 34, 167–339.
Sharp K.A. and Honig B. (1990) Annu. Rev. Biophys. Biophys. Chem., 19, 301–332.
Skelton N.J., Akke M., Kördel J., Thulin E., Forsén S. and Chazin W.J. (1992) FEBS Lett., 303, 136–140.
Spera S. and Bax A. (1991) J. Am. Chem. Soc., 113, 5490–5492.
Stockman B.J., Scahill T.A., Strakalaitis N.A., Brunner D.P., Yem A.W. and DeibelJr. M.R. (1992) J. Biomol. NMR, 2, 591–596.
Szilágyi L. and Jardetzky O. (1989) J. Magn. Reson., 83, 441–449.
Veerapandian B., Gilliland G.L., Raag R., Svensson A.L., Masui Y., Hirai Y. and Poulos T.L. (1992) Protein Struct. Funct. Genet., 12, 10–23.
Wang J., Hick A.P., Loh S.N., LeMaster D.M. and Markley J.L. (1992) Biochemistry, 31, 921–936.
Warshel A. and Aqvist J. (1991) Annu. Rev. Biophys. Biophys. Chem., 20, 267–298.
Weber P.L., Brown S.C. and Mueller L. (1987) Biochemistry, 26, 7282–7290.
Williamson M.P. (1990) Biopolymers, 29, 1423–1431.
Williamson M.P. and Asakura T. (1991) J. Magn. Reson., 94, 557–562.
Williamson M.P. and Asakura T. (1993) J. Magn. Reson. Ser. B, 101, 63–71.
Williamson M.P., Asakura T., Nakamura E. and Demura M. (1992) J. Biomol. NMR, 2, 83–98.
Wishart D.S., Sykes B.D. and Richards F.M. (1991) J. Mol. Biol., 222, 311–333.
Wishart D.S., Sykes B.D. and Richards F.M. (1992) Biochemistry, 31, 1647–1651.
Zhou N.E., Zhu B.-Y., Sykes B.D. and Hodges R.S. (1992) J. Am. Chem. Soc., 114, 4320–4326.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ösapay, K., Case, D.A. Analysis of proton chemical shifts in regular secondary structure of proteins. J Biomol NMR 4, 215–230 (1994). https://doi.org/10.1007/BF00175249
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00175249