Summary
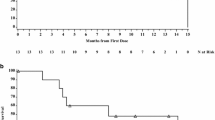
Epirubicin, a stereoisomer of doxorubicin with suggested lower potential for cardiotoxicity in experimental animal tumor systems, was studied in a disease-oriented phase II trial in patients with advanced colorectal cancer. The drug was given as a direct iv injection of 90 mg/m2 q 3 weeks. No objective response was observed in 52 evaluable patients with colon (n = 34) and rectal (n = 18) carcinoma. Fourteen patients (27%) had stable disease for a median of four treatment courses. Leukopenia (88%), nausea and vomiting (71%) and alopecia (54%) were the most common toxic effects.
We conclude that epirubicin at the present dose and schedule is an ineffective agent in patients with metastatic colorectal cancer.
Similar content being viewed by others
References
Kemeny N, Golbey R: A chemotherapeutic approach to colorectal carcinoma. In MW Stearns JR. (ed): Neoplasms of the Colon, Rectum and Anus. John Wilex and Sons, New York, 1980, pp 155–168
Arcamone F, Penco S, Vigevani A, Redaelli S, Franchi G, Di Marco A, Casazza AM, Dasdia T, Formelli F, Necco A, Soranco C: Synthesis and antitumor properties of new glycosides of daunomycinone and adriamycinone. J Med Chem 18:703–707, 1975
Giuliani FC, Kaplan NO: New doxorubicin analogs active against doxorubicin-resistant colon tumor xenografts in the nude mouse. Cancer Res 40:4682–4687, 1980
Casazza AM, Di Marco A, Bertazzoli C, Formelli F, Giuliani F, Pratesi G: Antitumor activity, toxicity and pharmacological properties of 4′ -epiadriamycin. In W Siegenthaler and R Lüthy (s): Current Chemotherapy, American Society for Microbiology, Washington, DC, USA, 1978, pp 1257–1260
Zbinden G, Brändle E: Toxicologic screening of daunorubicin (NSC-82151), adriamycin (NSC-123127) and their derivatives in rats. Cancer Chemother Rep 59:707–715, 1975
Casazza AM: Experimental evaluation of anthracycline analogs. Cancer Treat Rep 63:835–844, 1979
Broggini M, Colombo T, Martini A, Donelli MG: Studies on the comparative distribution of doxorubicin and 4′ -epidoxorubicin in mice and rats. Cancer Treat Rep 64:897–904, 1980
Villani FP, Favalli L, Piccinini F: Relationship between the effect on calcium turnover and early cardiotoxicity of doxorubicin and 4′ -epidoxorubicin in guinea pig heart muscle. Tumori 66:689–697, 1980
Natale N, Brambilla M, Luchini S, Martini A, Moro E, Paxxiarini MA, Vago G, Trabattoni A: 4 '-Epidoxorubicin and doxorubicin: toxicity and pharmakinetics in cancer patients. (Abstract) 12th Proc Internatl Congress Chemother, p. 114, 1981
Bonfante V, Bonadonna G, Villani F, Di Fronzo G, Martini A, Casazza AM: Preliminary phase I study of 4′ -epiadriamycin. Cancer Treat Rep 63:915–918, 1979
Bonfante V, Bonadonna G, Villani F, Martini A: Preliminary clinical experience with 4 ′-epidoxorubicin in advanced human neoplasia. Recent Results Cancer Res 74: 192–199, 1980
WHO Handbook for Reporting Results of Cancer Treatment, Geneva WHO, 1979
Ganzina F: 4′ -Epidoxorubicin, a new analogue of doxorubicin: a preliminry overview of preclinical and clinical data. Cancer Treat Rev 10:1–22, 1983
Michaelson R, Kemmeny N, Young Ch: Phase II evaluation of 4′ -epidoxorubicin in patients with advanced colorectal carcinoma. Cancer Treat Rep 66:1757–1758, 1982
Bodey G, Yap BS, Ajani J, Aboud A, Yap HY, Estey E: Clinical trials of 4′ -epidoxorubicin. In Cavalli F and Lenzhofer R (eds): New Anthracyclines in the Chemotherapy of Solid Tumors and Hematologic Malignancies. Proc 13th Internatl Congress Chemother, 1983, pp 27–30
Young CW, Wittes RE, Jain K, Casper ES: Clinical evaluation of 4′ -epidoxorubicin. In Cavalli F and Lenzhofer R (eds): New Anthracyclines in the Chemotherapy of Solid Tumors and Hematologic Malignancies. Proc 13th Internatl Congress Chemother, 1983, pp 17–20
Frytak S, Moertel CG, Schutt AJ, Hahn RG, Reitemeier RJ: Adriamycin (NSC-123127) therapy for advanced gastrointestinal cancers. Cancer Chemother Rep 59:405–409, 1975
Moertel CG, Schutt AJ, Hahn RG, Reitemerer RJ: Effects of patient selection on results of phase II chemotherapy trials in gastrointestinal cancer. Cancer Chemother Rep 58:257–259, 1974
Falkson G, Falkson HC, Vorobiof DA, Pretorius HL, Falkson CB: A phase II study of 4 t' -epidoxorubicin (IMI 28) in colorectal cancer. In Cavalli F and Lenzhofer R (eds): New Anthracyclines in the Chemotherapy of Solid Tumors and Hematologic Malignancies. Proc 13th Internatl Congress Chemotherapy, 1983, pp 35–39
Bonfante V, Villani F, Bonadonna G: Toxic and therapeutic activity of 4′ -epidoxorubicin. Tumori 68:105–111, 1982
Robustelli Delia Cuna G, Ganzina F, Tramarin R, Pavesi L: Phase II evaluation of 4′ -epidoxorubicin (Abstract), UICC Conference on Clinical Oncology, Lausanne (Switzerland), p. 45, 1981
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holdener, E.E., Hansen, H.H., Høst, H. et al. Epirubicin in colorectal cancer. Invest New Drugs 3, 63–66 (1985). https://doi.org/10.1007/BF00176826
Issue Date:
DOI: https://doi.org/10.1007/BF00176826