Summary
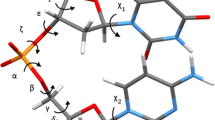
The DNA·DNA duplex \({\text{d}}\left( {{\text{CGCGTT}}_{{\text{SCH}}_{\text{2}} {\text{O}}} {\text{TTGCGC}}} \right)\)·d(GCGCAAAACGCG) (designated duplex III) containing a 3′-thioformacetal (3′-TFMA) linkage in the center of the sequence was characterized in detail by two- and three-dimensional homonuclear NMR spectroscopy. The NMR results were analyzed and compared with those of two duplexes of the same sequence: One is an unmodified reference sequence and the other contains a formacetal (OCH2O) linkage at the central T^T step (designated duplex I and duplex II, respectively). In general, the NMR spectra of duplex III closely resemble those of the analogous duplexes I and II, suggesting an overall B-type structure adopted by the 3′-TFMA-modified duplex III. Nonetheless, the detection of several distinct spectral features originating from the protons at the \({\text{T6}}_{{\text{3' - SCH}}_{\text{2}} {\text{O}}} {\text{T7}}\) modification site is indicative of a local conformation that is clearly different from the corresponding region in duplexes I and II. The 3′-thioformacetal linker, in contrast to the formacetal (FMA) linkage, cannot be accommodated in a conformation usually found in natural nucleic acid duplexes. As a consequence, the 3′-TFMA-modified T6 sugar adopts an O4′-endo form (an intermediate structure between the usual C2′-endo and C3′-endo forms). This change is accompanied by a change in the ε (C4′−C3′−S3′−CH2) dihedral angle and by subsequent adjustments of other torsion angles along the backbone. Notably, this conformational readjustment at the T6–T7 backbone linkage is localized; its collective result has negligible effect on base-base stacking of the T6 and T7 residues. A close examination of the COSY data in all three duplexes reveals a subtle variation in sugar geometry, with more S-type character adopted by the modified duplexes II and III. The results of this study illustrate that, although the difference between FMA and 3′-TFMA linkages is merely in the substitution of the T6(O3′) in the former by a sulfur atom in the latter, the stereoelectronic difference in a single atom can induce significant local structural distortion in an otherwise well-structured oligonucleotide duplex.
Similar content being viewed by others
References
Blommers, M.J., Van de Ven, F.J.M., Van der Marel, G.A. and Van Boom, J.H. (1991) Eur. J. Biochem., 210, 33–51.
Chandrasekaran, R., Birdsall, D.L., Leslie, A.G.W. and Ratcliff, R.L. (1980) Nature, 283, 743–745.
Chary, K.V.R., Hosur, R.V., Govil, G., Zu-Kun, T. and Miles, H.T. (1988) Biochemistry, 26, 1315–1322.
Cruse, W.B.T., Salisbury, S.A., Brown, T., Cosstick, R., Eckstein, F. and Kennard, O. (1986) J. Mol. Biol., 192, 891–905.
Dickerson, R.E. (1983) J. Mol. Biol., 166, 419–441.
Froehler, B.C., Ng, P.G. and Matteucci, M.D. (1986) Nucleic Acids Res., 14, 5399–5407.
Gao, X. and Burkhart, W. (1991) Biochemistry, 30, 7730–7739.
Gao, X., Brown, F.K., Jeffs, P., Bischofberger, N., Lin, K.-Y., Pipe, A.J. and Noble, S.A. (1992) Biochemistry, 31, 6228–6236.
Gao, X. and Jeffs, P.W. (1994) J. Biomol. NMR, in press.
Haasnoot, C.A.G., De Leeuw, F.A.A.M. and Altona, C. (1980) Tetrahedron, 36, 2783–2792.
Heinemann, U., Rudolph, L.-N., Claudia, A., Morr, M., Heikens, R.F. and Blocker, H. (1991) Nucleic Acids Res., 19, 427–433. This report describes a crystallographic study of a selfcomplementary DNA duplex \({\text{d}}\left( {{\text{GCCCG}}_{{\text{CH}}_{\text{2}} {\text{PO}}_{\text{2}} {\text{O}}} {\text{GGC}}} \right)_2 \), in which the 3′-oxygen is substituted by a methylene group. This duplex adopted a deformed A-type conformation with the exception of the modified dG5 which displays C2′-exo (N-type) rather than the C3′-endo conformation of the unmodified residue in the reference sequence. Interestingly, it was found that the restructuring in sugar pucker of the modified dG5 was accompanied by a crankshaft rotation of the α and γ torsion angles of the same residue (in the 5′-direction), resulting in an unusual α(trans)-β(trans)-γ(trans) backbone conformation.
Jones, R.J., Lin, K-Y., Milligan, J.F., Wadwani, S. and Matteucci, M.D. (1993) J. Org. Chem., 58, 2983–2991.
Katahira, M., Sugeta, H., Kyogoku, Y., Fujii, S., Fujisawa, R. and Tomita, K. (1988) Nucleic Acids Res., 16, 8619–8632.
Matteucci, M. (1990) Tetrahedron Lett., 31, 2385–2388.
Matteucci, M. (1991) Nucleosides and Nucleotides, 10, 231–234.
Matteucci, M., Lin, K.-Y., Butcher, S. and Moulds, C.J. (1991) J. Am. Chem. Soc., 113, 7767–7768.
McBride, L.J. and Caruthers, N.G. (1983) Tetrahedron Lett., 24, 245–248.
Neuhaus, D. and Williamson, M. (1989) The Nuclear Overhauser Effect in Structural and Conformational Analysis, VCH Publishers, New York, NY, pp. 104–110.
Patel, D.J., Shapiro, L. and Hare, D. (1987) Q. Rev. Biophys., 20, 35–112.
Reid, B.R. (1987) Q. Rev. Biophys., 20, 1–34.
Saenger, W. (1984) Principles of Nucleic Acid Structure, Springer Verlag, New York, NY.
Uhlmann, E. and Peyman, A. (1990) Chem. Rev., 90, 543–584.
Veal, J. and Brown, F.K. (1992) predicted that the 3′-TFMA-modified sugar prefers a C3′-endo-type conformation as opposed to a C2′-endo by ab-initio quantum mechanics, unpublished results.
Veeneman, G.H., Van der Marel, G.A., Van den Elst, H. and Van Boom, J.H. (1991) Tetrahedron, 47, 1547–1562.
Veeneman, G.H., Van der Marel, G.A., Van den Elst, H. and Van Boom, J.H. (1990) Recl. Trav. Chim. Pays-Bas, 109, 449–451.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, Wiley, New York, NY.
Author information
Authors and Affiliations
Additional information
Supplementary material available from the authors: One table containing J1′2′, J1′2″ and J3′4′ of duplexes I, II and III.
Rights and permissions
About this article
Cite this article
Gao, X., Jeffs, P.W. Unusual conformation of a 3′-thioformacetal linkage in a DNA duplex. J Biomol NMR 4, 17–34 (1994). https://doi.org/10.1007/BF00178333
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00178333