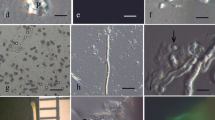
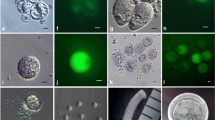
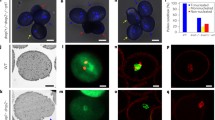
Summary
Sperm cells of pollen tubes grown both in vivo and in vitro form a male germ unit. Extensions from both sperm cells of each pollen tube are closely associated with the tube nucleus. A high yield (2.7 × 104. 20 mg−1 pollen grains germinated) of intact sperm cells was obtained following release by osmotic shock from pollen tubes grown in vitro. Structural integrity of isolated sperm was maintained by isolation at low temperature in an osmotically balanced medium. At 4° C many isolated sperm pairs were still enclosed within the pollentube inner plasma membrane. Sperm cells not enclosed within this membrane no longer remained connected as a pair. During isolation vesicles formed on the sperm cell surface from disruption of the fibrillar components bridging the periplasmic space. Both in the pollen tube and after isolation the sperm nucleus is in close association with at least one region of the sperm plasma membrane. Sperm isolated at room temperature showed the presence of nucleopores, and nuclei were euchromatic, instead of heterochromatic as in intact sperm in the pollen tube.
Similar content being viewed by others
References
Brewbaker JL, Kwack BH (1963) The essential role of calcium in pollen germination and tube growth. Am J Bot 50:859–865
Cass DD (1973) An ultrastructural and Nomarski interference study of the sperms of barley. Can J Bot 51:601–605
Cass DD, Fabi GC (1988) Structure and properties of sperm cells isolated from the pollen of Zea mays. Can J Bot 66:819–825
Chapman GP, Ainsworth CC, Chatham CJ (eds) (1988) Eukaryotic cell recognition. Concepts and model systems. Cambridge University Press, Cambridge
Charzynska M, Murgia M, Milanesi C, Cresti M (1989) Origin of sperm cell association in the “male germ unit” of Brassica pollen. Protoplasma 149:1–4
Cresti M, Murgia M, Theunis CH (1990) Microtubule organization in sperm cells in the pollen tubes of Brassica oleracea L. Protoplasma 154:151–156
De Waele M, De Mey J, Moeremans M, De Brabander M, Van Camp B (1983) Immunogold staining method for the detection of cell surface antigens with monoclonal antibodies. In: Bullock GR, Petrusz P (eds) Techniques in immunocytochemistry, vol 2. Academic Press, London, pp 1–23
Dumas C, Knox RB, McConchie CA, Russell SD (1984) Emerging physiological concepts in fertilization. What's New Plant Physiol 15:17–20
Dupuis I, Roeckel P, Matthys-Rochon E, Dumas C (1987) Procedure to isolate viable sperm cells from corn (Zea mays L) pollen grains. Plant Physiol 15:17–20
Emons AMC, Kron M, Knuiman B, Platel T (1988) Intramembrane particle pattern in vegetative and generative plasma membranes of lily pollen grain and pollen tube. In: Wilms HJ, Kleijzer CJ (eds) Plant sperm cells as tools for biotechnology. Pudoc, Wageningen, pp 41–48
Evans DE, Dungey SG, Grey I (1987) A new method for collection of high quality Brassica pollen. Cruciferae Newsl 12:62
Friend DS (1982) Plasma-membrane diversity in a highly polarized cell. J Cell Biol 93:243–249
Friend DS, Fawcett DW (1974) Membrane differentiation in freeze fracture mammalian sperm. J Cell Biol 63:641–664
Goding JW (1983) Monoclonal antibodies: principles and practise. Academic Press, London, pp 1–276
Heslop-Harrison J, Heslop-Harrison Y, Shivanna KR (1984) The evaluation of pollen quality and further appraisal of the fluorochromatic (FCR) test procedure. Theor Appl Genet 67:367–375
Hodgkin T, Lyon GD (1983) Detection of pollen germination inhibitors in Brassica oleracea tissue extracts. Ann Bot 52:781–789
Hough T, Bernhardt P, Knox RB, Williams EG (1985) Applications of fluorochromes to pollen biology. II. The DNA probes ethidium bromide and Hoechst 33258 in conjunction with the callose specific analine blue fluorochrome. Stain Tech 60:155–162
Hough T, Singh MB, Smart IJ, Knox RB (1986) Immunofluorescent screening of monoclonal antibodies to surface antigens of animal and plant cells bound to polycarbonate membranes. J Immunol Methods 92:103–107
Knox RB, Southworth D, Singh MB (1988) Sperm cell determinants and control of fertilization in plants. In: Chapman GP, Ainsworth CC, Chatham CJ (eds) Eukaryotic cell recognition. Concepts and model systems. Cambridge University Press, Cambridge, pp 175–193
Lindforss B (1896) Zur Biologie des Pollens. Jahr Wissen Bot 29:1–38
Matthys-Rochon E, Vergne P, Detchepare S, Dumas C (1987) Male germ unit isolation from three tricellular pollen species: Brassica oleracea, Zea mays and Triticum aestivum. Plant Physiol 83:464–466
McConchie CA, Jobson S, Knox RB (1985) Computer-assisted reconstruction of the male germ unit in pollen of Brassica campestris. Protoplasma 127:57–63
McConchie CA, Russell SD, Dumas C, Tuohy M, Knox RB (1987) Quantitative cytology of the sperm cells of Brassica campestris and B. oleracea. Planta 170:446–452
Mogensen HL, Wagner VT, Dumas C (1990) Quantitative, threedimensional ultrastructure of isolated corn (Zea mays) sperm cells. Protoplasma 153:136–140
Mulcahy GB, Mulcahy DL (1988) The effect of supplemented media on the growth in vitro of biand tri-nucleate pollen. Plant Sci 55:213–216
Pennell RI, Geltz NR, Koren E, Russell SD (1987) Production and partial characterization of hybridoma antibodies elicited to the sperm of Plumbago zeylanica. Bot Gaz 148:401–406
Phelps BM, Koppel DE, Primakoff P, Myles DG (1990) Evidence that proteolysis of the surface is an initial step in the mechanism of formation of sperm cell surface domains. J Cell Biol 111:1839–1847
Raghavan V (1987) Developmental strategies of the angiosperm pollen: a biochemical perspective. Cell Differ 21:213–226
Roeckel P, Chaboud A, Matthys-Rochon E, Russell S, Dumas C (1990) Sperm cell structure, development and organization. In: Blackmore S, Knox RB (eds) Microspores: evolution and ontogeny. Academic Press, London, pp 281–307
Roman H (1948) Directed fertilization in maize. Proc Natl Acad Sci USA 34:36–42
Russell SD (1984) Ultrastructure of the sperm of Plumbago zeylanica. II. Quantitative cytology and three-dimensional organization. Planta 162:385–391
Russell SD (1985) Preferential fertilization in Plumbago: ultrastructural evidence for gamete level recognition in an angiosperm. Proc Natl Acad Sci USA 82:6129–6132
Russell SD (1986) Isolation of sperm cells from the pollen of Plumbago zeylanica. Plant Physiol 81:317–319
Russell SD, Cass DD (1983) Unequal distribution of plastids and mitochondria during sperm formation in Plumbago zeylanica. In: Mulcahy DL, Ottaviano E (eds) Pollen. Biology and implications for plant breeding. Elsevier, Amsterdam, New York, pp 135–140
Shivanna KR, Xu H, Taylor P, Knox RB (1988) Isolation of sperms from the pollen tubes of flowering plants during fertilization. Plant Physiol 87:647–650
Singer SJ, Nicholson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Southworth D (1986) Sperm cell structure in Gerbera jamesonii. (Asteraceae). In: Williams EG, Knox RB, Irvine D (ed) Pollination '86. School of Botany. University of Melbourne, pp 172–177
Southworth D (1990) Membranes of sperm and vegetative cells in pollen of Gerbera jamesonii. J Struct Biol 103:97–103
Southworth D, Knox RB (1988) Methods for isolation of sperm cells from pollen. In: Wilms HJ, Keijzer CJ (eds) Plant sperm cells as tools for biotechnology. Pudoc, Wageningen, pp 87–95
Southworth D, Knox RB (1989) Flowering plant sperm cells: isolation from pollen of Gerbera jamesonii (Asteraceae). Plant Sci 60:273–277
Southworth D, Platt-Aloia KA, Thompson WW (1988) Freeze fracture of sperm and vegetative cells in Zea mays pollen. J Ultrastruct Res 101:165–172
Southworth D, Platt-Aloia KA, De Mason DA, Thompson WW (1989) Freeze-fracture of the generative cell of Phoenix dactylifera (Arecaceae). Sex Plant Reprod 2:270–276
Spurr A (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Staff IA, Taylor P, Kenrick J, Knox RB (1989) Ultrastructural analysis of plastids in angiosperm pollen tubes. Sex Plant Reprod 2:70–76
Taylor P, Kenrick J, Li Y, Kaul V, Gunning BES, Knox RB (1990) The male germ unit of Rhododendron: quantitative cytology, three-dimensional reconstruction, isolation and detection using fluorescent probes. Sex Plant Reprod 2:254–264
Van Aelst AC, Theunis CH, Van Went JL (1990) Freeze-facture studies on isolated sperm cells of Spinacia oleracea L. Protoplasma 153:204–207
Wagner V, Mogensen L (1987) The male germ unit in the pollen and pollen tubes of Petunia hybrida. Am J Bot 73:645–646
Wagner VT, Dumas C, Mogensen HL (1989) Morphometric analysis of isolated Zea mays sperm. J Cell Sci 93:179–184
Wilms HJ (1986) Dimorphic sperm cells in the pollen grain of Spinacia. In: Cresti M, Dallai R (eds) Biology of reproduction and motility in plants and animals. University of Siena, Siena, pp 193–198
Wilms HJ, Aelst AC (1983) Ultrastructure of spinach sperm cells in mature pollen. In: Erdelska O (ed) Fertilization and embryogenesis in ovulated plants. Czech Centre Biol Ecol Sci, Bratislava, pp 105–112
Zhou C, Zee SY, Yang HY (1990) Microtubule organization of in situ and isolated generative cells in Zephyranthes grandiflora Lindl. Sex Plant Reprod 3:213–218
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taylor, P.E., Kenrick, J., Blomstedt, C.K. et al. Sperm cells of the pollen tubes of Brassica: Ultrastructure and isolation. Sexual Plant Reprod 4, 226–234 (1991). https://doi.org/10.1007/BF00190009
Issue Date:
DOI: https://doi.org/10.1007/BF00190009