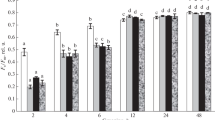
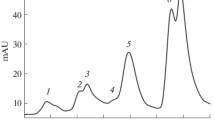
Abstract
The effects of varying the steady-state rate of non-cyclic photosynthetic electron transport on the leaf adenylate energy charge and the epoxidation state of the xanthophyll-cycle pigments were determined in leaves of cotton (Gossypium hirsutum L.) and the mangrove (Aegialitis annulata R.Br.). Different photosynthetic rates were obtained by varying the intercellular CO2 concentration and/or the leaf temperature, and in some cases, by changing the leaf conductance to CO2 diffusion. Also determined were the effects of these treatments on the changes in the adenylate energy charge and the epoxidation state of the xanthophyll-cycle pigments that occur after darkening of the leaves. The leaf adenylate pool remained close to equilibrium with the adenylate kinase both in the light at steady state and during dark relaxation. The adenylate energy charge increased as the photosynthetic rate decreased and maximal levels were obtained when CO2 assimilation and, therefore, non-cyclic electron flow were maximally inhibited. This implies that, in nature, photophosphorylation may provide energy needed for ion-pumping and biosynthetic and repair processes, even under stress conditions that severely restrict or prevent photosynthetic gas exchange. High levels of de-epoxidized violaxanthin in the light did not necessarily indicate or depend on a high adenylate energy charge. Dithiothreitol, an inhibitor of the violaxanthin de-epoxidase a nd ascorbate peroxidase, did not inhibit the adenylate energy charge in the light. Thus we conclude that coupled electron transport during inhibited CO2 fixation was not driven by a dithiothreitol-sensitive Mehler ascorbate-peroxidase reaction. The changes in the adenylate energy charge and xanthophyll re-epoxidation that follow when leaves were darkened are strongly affected by the preceding photosynthetic rate. Postillumination fluctuations in adenylate energy charge, both at 15 ° and 27 °C, were most pronounced when the preceding photosynthetic rate was minimal and least pronounced when this rate was maximal. Temperature had a considerably greater influence in the dark on xanthophyll re-epoxidation than on the pattern of adenylate relaxation.
Similar content being viewed by others
Abbreviations
- A:
-
antheraxanthin
- adenylate kinase:
-
(myokinase), ATP: AMP phosphotransferase
- Ci :
-
intercellular CO2 concentration
- DPS:
-
de-epoxidation state of violaxanthin, ([Z + A]/[V + A + Z])
- ΔpH:
-
trans-thylakoid proton gradient
- ε:
-
[2ATP+ADP]
- PET:
-
photosynthetic electron transportrate
- PFD:
-
photon flux density
- ∑:
-
[ATP+ADP+AMP]
- V:
-
violaxanthin
- Z:
-
zeaxanthin
References
Andersen, R.A., Sowers, J.A. (1968) Optimum conditions for bonding of plant phenols to insoluble polyvinylpyrrolidone. Phyto chemistry 7, 293–301
Avron, M., Schreiber, U. (1977) Proton gradients as possible inter mediary energy transducers during ATP-driven reverse electron flow in chloroplasts. FEBS Lett. 77, 1–6
Ball, M. (1986) Photosynthesis in mangroves. Wetlands (Australia) 6, 12–22
Bilger, W., Björkman, O. (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 25, 173–185
Bilger, W., Björkman, O. (1991) Temperature dependence of violax anthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 184, 226–234
Bilger, W., Björkman, O., Thayer, S.S. (1989) Light-induced spectral absorbance changes in relation to photosynthesis and the epox idation state of xanthophyll cycle components in cotton leaves. Plant Physiol. 91, 542–551
Björkman, O., Demmig, B. (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 170, 489–504
Björkman, O., Demmig-Adams, B. (1993) Regulation of photosynthetic light energy capture, conversion and dissipation in leaves of higher plants. In: Ecological studies, vol. 100, Schulze, E.-D., Caldwell, M., eds., Springer-Verlag, (in press)
Björkman, O., Demmig, B., Andrews, T.J. (1988) Mangrove photosynthesis: Response to high-irradiance stress. Aust. J. Plant Physiol. 15, 43–61
Bomsel, J.-L., Pradet, A. (1967) Étude des adénosine-5′-mono, di et tri-phosphates dans les tissus végétaux II. Évolution in vivo de l'ATP, l'ADP et l'AMP dans les feuilles de blé en fonction de différentes conditions de milieu. Physiol. Vég. 5, 223–236
Bomsel, J.-L., Pradet, A. (1968) Study of adenosine 5′-mono, di- and triphosphates in plant tissues IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: Theoretical and experimental study. Biochim. Biophys. Acta 162, 230–242
Brugnoli, E., Björkman, O. (1992) Growth of cotton under continuous salinity stress: influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta 187, 335–347
Chapman, A.G., Atkinson, D.E. (1973) Stabilization of adenylate energy charge by the adenylate deaminase reaction. J. Biol. Chem. 248, 8309–8312
Demmig-Adams, B., Adams, W.W. III (1992) Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 599–626
Demmig-Adams, B., Adams, W.W. III., Heber, U., Neimanis, S., Winter, K., Krüger, A., Czygan, F.-C., Bilger, W., Björkman, O. (1990) Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 92, 293–301
Foyer, C.H., Dujardyn, M., Lemoine, Y. (1989) Responses of photosynthesis and the xanthophyll and ascorbate-glutathione cycles to changes in irradiance, photoinhibition and recovery. Plant Physiol. Biochem. 27, 751–760
Gamon, J.A., Field, C.B., Bilger, W., Björkman, O., Fredeen, A.L., Peñuelas, J. (1990) Remote sensing of the xanthophyll cycle and chlorophyll fluorescence in sunflower leaves and canopies. Oecologia 85, 1–7
Gamon, J.A., Peñuelas, J., Field, C.B. (1992) A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 41, 35–44
Gilmore, A.M., Björkman, O. (1994) Adenine nucleotides and the xanthophyll cycle in leaves. II. Comparison of the effects of CO2 and temperature-limited photosynthesis on photosystem II fluorescence quenching, the adenylate energy charge and violaxanthin de-epoxidation in cotton. Planta 192, 537–544
Gilmore, A.M., Yamamoto, H.Y. (1992) Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching me diated by ATP. Proc. Natl. Acad. Sci. USA 89, 1899–1903
Gilmore, A.M., Yamamoto, H.Y. (1993) Zeaxanthin-dependent quenching of the variable fluorescence arising from ATP-induced reverse electron flow. In: Research in photosynthesis, vol. I, pp. 255–258, Murata, N. ed., Dordrecht: Kluwer Academic Publishers
Hager, A. (1967) Untersuchungen über die Rückreaktionen im Xanthophyll-Cyclus bei Chlorella, Spinacia und Taxus. Planta 76, 138–148
Hager, A. (1969) Lichtbedingte pH-Erniedrigung in einem Chloroplasten-Kompartiment als Ursache der enzymatischen Violaxanthin → Zeaxanthin-Umwandlung; Beziehungen zur Photophosphorylierung. Planta 89, 224–243
Hager, A. (1980) The reversible, light-induced conversions of xanthophylls in the chloroplast. In: Pigments in plants, pp. 57–79, Czygan, F.C. ed., Fischer, Stuttgart
Heber, U. (1969) Conformational changes of chloroplasts induced by illumination of leaves in vivo. Biochim. Biophys. Acta 180, 302–319
Heber, U. (1973) Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim. Biophys. Acta 305, 140–152
Heber, U. (1974) Metabolite exchange between chloroplasts and cytoplasm. Annu. Rev. Plant Physiol. 25, 393–421
Heber, U., Santarius, K.A. (1970) Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z. Naturforsch. 25b, 718–728
Heber, U., Walker, D.A. (1992) Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol. 100, 1621–1626
Kobayashi, Y., Köster, S., Heber, U. (1982) Light scattering, chlorophyll fluorescence and state of the adenylate system in illuminated spinach leaves. Biochim. Biophys. Acta 682, 44–54
Neubauer, C. (1992) DTT-effect on non-photochemical fluorescence quenching and ascorbate-peroxidase and violaxanthin de-epoxidase activity. (Abstr.) Plant Physiol. 99, Suppl., 151
Neubauer, C., Yamamoto, H.Y. (1992) Mehler-peroxidase reaction mediates zeaxanthin formation and zeaxanthin-related fluorescence quenching in intact chloroplasts. Plant Physiol. 99, 1354–1361
Pfündel, E.E., Dilley, R.A. (1993) The pH dependence of violaxanthin deepoxidation in isolated pea chloroplasts. Plant Physiol. 101, 65–71
Schäfer, C., Björkman, O. (1989) Relationship between efficiency of photosynthetic energy conversion and chlorophyll fluorescence quenching in upland cotton (Gossypium hirsutum L.). Planta 178, 367–376
Schreiber, U. (1980) Light-activated ATPase and ATP-driven reverse electron transport in intact chloroplasts. FEBS Lett. 122, 121–124
Schreiber, U., Avron, M. (1979) Properties of ATP-driven reverse electron flow in chloroplasts. Biochim. Biophys. Acta 546, 436–447
Schreiber, U., Neubauer, C. (1990) O2-dependent electron flow, membrane energization and the mechanism of non-photochemical quenching of chlorophyll fluorescence. Photosynth. Res. 25, 279–293
Schreiber, U., Reising, H., Neubauer, C. (1991) Contrasting pH-optima of light-driven O2- and H2O2-reduction in spinach chloroplasts as measured via chlorophyll fluorescence quenching. Z. Naturforsch. 46c, 635–643
Sellami, A. (1976) Évolution des adenosine phosphates et de la charge énergétique dans les compartiments chloroplastique et nonchloroplastique des feuilles de blé. Biochim. Biophys. Acta 423, 524–539
Sellami, A., Bomsel J.-L. (1975) Évolution de la charge énergétique du pool adénylique des feuilles de Blé au cours de l'anoxie. Étude de la réversibilité des phénomènes observés. Physiol. Vég. 13, 611–617
Siebke, K., Laisk, A., Oja, V., Kiirats, O., Raschke, K., Heber, U. (1990) Control of photosynthesis in leaves revealed by rapid gas exchange and measurements of the assimilatory force FA. Planta 182, 513–522
Thayer, S.S., Björkman, O. (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth. Res. 23, 331–343
Winter, K., Königer, M. (1989) Dithiothreitol, an inhibitor of violaxanthin de-epoxidation, increases the susceptibility of leaves of Nerium oleander L. to photoinhibition of photosynthesis. Planta 180, 24–31
Yabuki, N., Ashihara, H. (1992) AMP deaminase and the control of adenylate catabolism in suspension-cultured Catharanthus roseus cells. Phytochemistry 31, 1905–1909
Yamamoto, H.Y. (1962) Studies on the light and dark interconversions of leaf xanthophylls. Arch. Biochem. Biophys. 97, 168–173
Yamamoto, H.Y. (1979) Biochemistry of the violaxanthin cycle in higher plants. Pure Appl. Chem. 51, 639–648
Yin, Z.-H., Heber, U., Raghavendra, A.S. (1993) Light-induced pH changes in leaves of C4 plants. Comparison of cytosolic alkalization and vacuolar acidification with that of C3 plants. Planta 189, 267–277
Author information
Authors and Affiliations
Additional information
We thank Connie Shih for skillful assistance in growing plants and for conducting HPLC analyses. A Carnegie Institution Fellowship to A.G. is also gratefully acknowledged. This manuscript is C.I.W.-D.PB. Publication No. 1183.
Rights and permissions
About this article
Cite this article
Gilmore, A.M., Björkman, O. Adenine nucleotides and the xanthophyll cycle in leaves. Planta 192, 526–536 (1994). https://doi.org/10.1007/BF00203591
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00203591