Summary
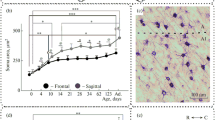
Changes in the expression of glial fibrillary acidic protein by astrocytes in the primary visual cortex of adult albino rats were analyzed with immunohisto-chemistry after unilateral destruction of the dorsal lateral geniculate nucleus. An increase in number of glial fibrillary acidic protein-immunoreactive astrocytes could be detected in the visual cortex of the side ipsilateral to the lesion in the short-term survival group (7–11 days post lesion), but this increase was extremely reduced after a postlesional survival time of 150 days. The quantitation of the glial response by image analysis showed, that the initial increase was mainly localized in the cortical layers II–IV, where the geniculo-cortical input terminates. The transient nature of this process was revealed by the measurements in the long-term survival group, where differences between experimental and control sides were substantially reduced. We conclude, that the remote glial response in the visual cortex is transient and that its disappearance indicates the end of a postlesional adaptation period in the neuropil.
Similar content being viewed by others
References
Aquino DA, Chiu F-C, Brosman CF, Norton WT (1988) Glial fibrillary acidic protein increases in the spinal cord of Lewis rats with acute experimental autoimmune encephalomyelitis. J Neurochem 5:1085–1096
Bignami A, Dahl D (1976) The astroglial response to stabbing. Immunofluorescence studies with antibodies to astrocyte-specific protein (GFAP) in mammalian and submammalian vertebrates. Neuropathol Appl Neurobiol 2:99–110
Bignami A, Eng LF, Dahl D, Uyeda CT (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 43:429–435
Bignami A, Dahl D, Rueger DC (1980) Glial fibrillary acidic (GFAP) protein in normal neural cells and in pathological conditions. Adv Cell Neurobiol 1:286–310
Brock TO, O'Callaghan JP (1987) Quantitative changes in the synaptic vesicle proteins synapsin 1 and P38 and the astrocyte-specific protein glial fibrillary acidic protein are associated with chemically-induced injury to the rat central nervous system. J Neurosci 7:931–942
Cortez SC, McIntosh TK, Noble LF (9189) Experimental fluid percussion brain injury. Vascular disruption and neuronal and glial alterations. Brain Res 482:271–282
Eng LF (1985) Glial fibrillary acidic protein (GFAP): the major protein of the glial intermediate filaments in differentiated astrocytes. J Neuroimmunol 8:203–214
Eng LF, Vanderhaegen JJ, Bignami A, Gerstl B (1971) An acidic protein isolated from astrocytes. Brain Res 28:351–354
Goebel HH, Schlie M, Eng LF (1987) Immunopathologic spectrum of the glial fibrillary acidic protein in neurooncology. Acta Histochem [Suppl] (Jena) 34:81–93
Graeber MB, Kreutzberg GW (1986) Astrocytes increase in glial fibrillary acidic protein during retrograde changes of facial motor nucleus. J Neurocytol 15:363–373
Hajós F, Bascó E (1984) The surface-contact glia. Adv Anat Embrol Cell Biol 84:1–81
Hajós F, Kálmán M (1989) Distribution of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in the rat brain. II. Mesencephalon, rhombencephalon and spinal cord. Exp Brain Res 78:164–173
Hajós F, Kálmán M, Zilles K, Schleicher A, Sotonyi P (1990) Remote astrocytic response as demonstrated by glial fibrillary acidic protein immunohistochemistry in the visual cortex of dorsal lateral geniculate nucleus lesioned rats. Glia 3:301–310
Hozumi I, Chiu F-C, Norton WT (1990) Biochemical and immuno-cytochemical changes in glial fibrillary acidic protein after stab wounds. Brain Res 524:64–71
Janeczko K (1989) Spatiotemporal patterns of the astroglial proliferation in rat brain injured at the postmitotic stage of postnatal development: a combined immunocytochemical and autoradiographic study. Brain Res 485:236–243
Kálmán M, Hajós F (1989) Distribution of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in the rat brain. I. Forebrain. Exp Brain Res 78:147–163
Liesi P, Dahl D, Vaheri A (1983) Laminin is produced by early rat astrocytes in primary culture. J Cell Biol 96:920–924
Ling EA, Leblond CP (1973) Investigation of glial cells in semithin sections. II. Variation with age in the numbers of the various glial cell types in rat cortex and corpus callosum. J Comp Neurol 149:73–81
Rosenfeld A, Kak AC (1976) Digital picture processing. Academic Press, New York
Schleicher A, Zilles K, Wree A (1986) A quantitative approach to cytoarchitectonics: Software and hardware aspects of a system for the evaluation and analysis of structural inhomogeneitis in nervous tissue. J Neurosci Methods 18:221–235
Schober W, Winkelmann E (1977) Die geniculocorticale Projektion bei Albinoratten. J Hirnforsch 18:1–20
Sefton AJ, Dreher B (1985) Visual system. In: Paxinos G (ed) The rat nervous system, vol 1. Academic Press, Sydney, pp 169–221
Tetzlaff W, Graeber MB, Bisby MA, Kreutzberg GW (1988) Increased glial fibrillary acidic protein synthesis in astrocytes during retrograde reaction of the rat facial nucleus. Glia 1:90–95
Vacca LL, Nelson SR (1984) Glial cells in Huntington's disease. Adv Cell Neurobiol 5:221–241
Vacca-Galloway LL (1986) Astrocytes in Huntington's chorea. In: Fedoroff S, Vernadakis A (eds) Astrocytes, vol 3. Academic Press, New York, pp 357–386
Zilles K (1985) The cortex of the rat. A stereotaxic atlas. Springer, Berlin, 121 pp
Zilles K, Hajós F, Kálmán M, Schleicher A (1991a) Mapping of glial fibrillary acidic protein-immunoreactivity in the rat forebrain and mesencephalon by computerized image analysis. J Comp Neurol 308:340–355
Zilles K, Kálmán M, Hajós F, Schleicher A (1991b) Developmental gradients of vasoactive intestinal polypeptide (VIP)-containing neurons in the rat visual cortex detected by image analysis. Dev Brain Res 60:137–144
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kálmán, M., Csillag, A., Schleicher, A. et al. Long-term effects of anterograde degeneration on astroglial reaction in the rat geniculo-cortical system as revealed by computerized image analysis. Anat Embryol 187, 1–7 (1993). https://doi.org/10.1007/BF00208191
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00208191