Abstract
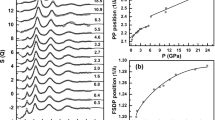
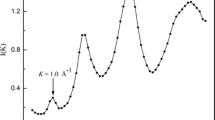
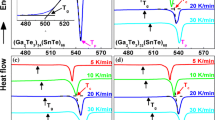
Relative-enthalpy measurements have been made on the hexagonal, tetragonal, glass and liquid phases of GeO2. The glass transition is very sensitive to the impurity content, with a T g ranging from 980 K for a pure product to 780 K for a Li-doped sample with 0.06 mol % Li. The relative C p change at T g of about 5% increases with the impurity content as a result of lower glass transition temperatures. Above 298 K the derived heat capacities are similar for all forms, with slightly higher values for the amorphous phases and two C p cross-overs at 400 and 1000 K between the hexagonal and tetragonal modifications. For both GeO2 and SiO2 the coordination state markedly affects C p and the entropy below 300 K, where the properties are much lower for the tetragonal than for the hexagonal modifications, i.e., S 298 = 39.7 vs 55.3 J/mole K and 27.8 vs 41.4 J/ mole K for GeO2 and SiO2, respectively. The high-temperature C p's of coesite and stishovite are likely similar to those of the low-pressure SiO2 forms. Finally, these results, low-temperature C p data and enthalpy-of-solution measurements have been used to derive a consistent set of thermodynamic properties for the GeO2 modifications.
Similar content being viewed by others
References
Akaogi M, Navrotsky A (1984) The quartz-coesite-stishovite transformations: new calorimetric measurements and calculation of phase diagrams. Phys Earth Planet Int 36:124–134
Andon RJL, Mills KC (1971) The heat capacity of tetragonal germanium dioxide. J Chem Thermodyn 3:583–587
Angell CA, Tucker JC (1974) Glass-forming molten-salt systems. In: Jeffes JHE, Tait RJ (eds) Physical chemistry of proces metallurgy. The Richardson conference, Inst Mining Metall Publ, London, pp 207–214
Herman RG (1988) Internally consistent thermodynamic data for minerals in the system Na2O-K2O-CaO-MgO-FeO -Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2. J Petrol 29:445–522
Bills JL, Cotton FA (1964) The heat of formation of germanium dioxide. J Phys Chem, 68:802–806
Counsell JF, Martin JF (1967) The entropy of tetragonal germanium dioxide. J Chem Soc (A) 560–561
Faktor MM, Carasso JI (1965) Tetragonal germanium dioxide and equilibria in the Ge-O-H system. J Electrochem Soc 112:817–822
Fontana EH, Plummer WA (1966) A study of viscosity-temperature relationships in the GeO2-SiO2 systems. Phys Chem Glasses 7:139–146
Gross P, Hayman C, Bingham JT (1966) Heats of formation of germanium tetrafluoride and of the germanium dioxides. Trans Faraday Soc 62:2388–2394
Hill VG, Chang LLY (1968) Hydrothermal investigation of GeO2. Am Mineral 53:1744–1748
Holm JL, Kleppa OJ, Westrum EF, Jr (1967) Thermodynamics of polymorphic transformations in silica. Thermal properties from 5 to 1070° K and pressure temperature stability fields for coesite and stishovite Geochim Cosmochim Acta 31:2289–2307
Kelley KK, Christensen AU (1961) High-temperature heat contents and entropies of crystalline and glassy germanium dioxide. U.S. Bureau Mines Rept Inv 5710
Kieffer SW (1982) Thermodynamics and lattice vibrations of minerals: 5. Applications to phase equilibria, isotopic fractionation, and high-pressure thermodynamic properties. Rev Geophys Space Phys 20:827–849
King EG (1958) Low-temperature heat capacities and entropies at 298.15° K of some oxides of gallium, germanium, molybdenum and niobium. J Am Chem Soc 80:1799–1800
Konnert JH, Karle J, Ferguson GA (1973) Crystalline ordering in silica and germania glasses. Science 179:177–179
Laubengayer AW, Morton DS (1932) Germanium. XXXIX. The polymorphism of germanium dioxide. J Am Chem Soc 54:2303–2320
Leko VK (1979) Viscosity of vitreous silica. Fiz Khim Stekla 5:258–278
Mah AD, Adami LH (1962) Heats and free energies of formation of germanium dioxide. U.S. Bureau Mines Rept Inv 6034
Majumdar AJ, Roy R (1965) Test of the applicability of the Clapeyron relationship to a few cases of solid-solid transitions. J Inorg Nucl Chem 27:1961–1973
Navrotsky A (1971) Enthalpies of transformation among the tetragonal, hexagonal, and glassy modifications of GeO2. J Inorg Nucl Chem 33:1119–1124
Newns GR, Hanks R (1966) Thermal behaviour of germanium dioxide. J Chem Soc (A) 954–957
Riebet P (1984) Viscosity and configurational entropy of silicate melts. Geochim Cosmochim Acta 48:471–483
Riebet P (1988) Superheating, melting and vitrification through decompression of high-pressure minerals. Nature 331:56–58
Richet P, Bottinga Y (1980) Heat capacity of silicate liquids: new measurements on NaAlSi3O8 and K2Si4O9. Geochim Cosmochim Acta 44:1535–1541
Richet P, Bottinga Y (1984a) Glass transitions and thermodynamic properties of amorphous SiO2, NaAlSinO2n+2 and KAlSi3O8. Geochim Cosmochim Acta 48:453–470
Richet P, Bottinga Y (1984b) Anorthite, andesine, diopside, wollastonite, cordierite, and pyrope: thermodynamics of melting, glass transitions, and properties of the amorphous phases. Earth Planet Sci Lett 67:415–432
Richet P, Bottinga Y (1986) Thermochemical properties of silicate glasses and liquids: a review. Rev Geophys 24:1–25
Richet P, Bottinga Y, Deniélou L, Petitet JP, Téqui C (1982) Thermodynamic properties of quartz, cristobalite and amorphous SiO2: drop calorimetry measurements from 1000 to 1800 K and a review from 0 to 2000 K. Geochim Cosmochim Acta 46:2639–2658
Robie RA, Hemingway BS, Fisher JR (1979) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. U.S. Geol Survey Bull 1452
Ross NL, Akaogi M, Navrotsky A, Suzaki JI, McMillan P (1986) Phase transitions among the CaGeO3 polymorphs (wollastonite, garnet, and perovskite structures): studies by high-pressure synthesis, high-temperature calorimetry and vibrational spectroscopy and calculation. J Geophys Res 91:4685–4696
Sarver JF (1961) Polymorphism and subsolidus equilibria in the system GeO2-TiO2. Am J Sci 259:709–718
Shelby JE (1975) Thermal expansion of mixed-alkali germanate glasses. J Appl Phys 46:193–196
Takahashi K, Yoshio T, Maruoka K (1974) Solution calorimetric approach to the structure of alkali germanate glasses. Yogyo Kyokai Shi 82:193–201
Vergano PJ, Uhlmann DR (1970) Crystallisation kinetics of germanium dioxide: the effect of stoichiometry on kinetics. Phys Chem Glasses 11:39–45
Watanabe H (1982) Thermochemical properties of synthetic high-pressure compounds relevant to the Earth's mantle. In: Akimoto S, Manghnani MH (eds) High-Pressure Research in Geo-physics, Center for Academic Publications, Tokyo, pp 81–90
Yonemura M, Kotera Y (1977) Kinetic study of the phase transformation of germanium dioxide. In: Wood J, Lindqvist O, Helgesson C (eds), Reactivity of solids, Plenum Press, New York, pp 227–232
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richet, P. GeO2 vs SiO2: Glass transitions and thermodynamic properties of polymorphs. Phys Chem Minerals 17, 79–88 (1990). https://doi.org/10.1007/BF00209228
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00209228