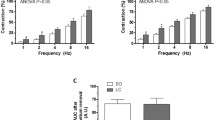
Summary
Neuropeptide Y (NPY)-immunoreactive (IR) nerve fibres were found around both arteries and veins and in smooth muscle trabeculae of the cat spleen with the highest density on the arterial side. Considerably more tyrosine hydroxylase (TH)- and dopamine-β-hydroxylase (DBH)-positive than NPY-IR nerves were seen in the trabeculae and splenic capsule. The NPY-IR nerves in the spleen most likely originated in the coeliac ganglion, since (1) splanchnic nerve sectioning did not change the splenic NPY-IR nerves, (2) most neurones in the coeliac ganglion were NPY-IR, as well as DBH- and TH-positive, and (3) NPY-IR was transported axonally from the coeliac ganglion towards the spleen via the splenic nerve. Local NPY infusion in the isolated, blood-perfused cat spleen caused a marked increase in splenic vascular resistance and a small volume reduction. NA caused a comparatively larger reduction in splenic volume than NPY in addition to vasoconstriction. VIP-IR cell bodies in the coeliac ganglion were NPY- and TH-negative. VIP-IR nerves were seen both around the splenic artery and vein as well as around arterioles and within venous trabeculae of the spleen. VIP infusion caused reduction of splenic perfusion pressure (i.e. vasodilation) as well as an increase in splenic volume. Substance P-IR nerves, most likely of splanchnic afferent origin, were present in the coeliac ganglion around the splenic artery and arterioles of the spleen. Infusion of substance P induced marked reduction in perfusion pressure and a reduction in splenic volume. Enkephalin-immunoreactive nerves of splanchnic origin surrounded some TH- and NPY-positive, coeliac ganglion cells.
It is concluded that several vasoactive peptides are located in splenic nerves. NPY is present in noradrenergic neurones and causes mainly increased vascular resistance. VIP occurs in non-adrenergic neurones of sympathetic origin and induces vasodilation and relaxation of the capsule. Finally, substance P is present in peripheral branches of spinal afferent nerves and causes vasodilation and capsule contraction. Stimulation of the splenic nerves may thus release several vasoactive substances in addition to noradrenaline, exerting a variety of actions.
Similar content being viewed by others
References
Blakely AGH, Brown C, Dearnaley DP, Woods RI (1969) Perfusion of the spleen with blood containing prostaglandin E1: transmitter liberation and uptake. Proc R Soc Lond B 174:281–292
Brandon KW, Rand MJ (1961) Acetylcholine and the sympathetic innervation of the spleen. J Physiol 157:18–32
Brown GL, Gillespie JS (1957) The output of sympathetic transmitter from the spleen of the cat. J Physiol 138:81–102
Brown CL, Davies BN, Ferry CR (1961) The effect of neuronal rest on the output of sympathetic transmitter from the spleen. J Physiol 159:365–380
Burn JH, Rand MJ (1960) Sympathetic postganglionic cholinergic fibres. Br J Pharmacol 15:56–66
Consolo S, Garattini S, Landinsky H, Thoenen H (1972) Effect of chemical sympathectomy on the content of acetylcholine, choline and choline acetyltransferase activity in the cat spleen and iris. J Physiol 220:639–646
Cripps H, Dearnaley DP (1972) Vascular responses and noradrenaline overflows in the isolated, blood-perfused cat spleen: Some effects of cocaine, normetanephrine and α-blocking agents. J Physiol 227:647–664
Cuello AC, Galfree G, Milstein C (1979) Detection of substance P in central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 76:3532–3536
Dahlsgaard C-J, Hökfelt T, Elfvin L-G, Skriboll L, Emson P (1982) Substance P containing primary sensory neurons projecting to the inferior mesenteric ganglion: evidence from combined retrograde tracing and immunohistochemistry. Neuroscience 7:647–652
Dale HH, Dudley HW (1929) The presence of histamine and acetylcholine in the spleen of the ox and horse. J Physiol (Lond) 68:97–113
De Burgh-Daly M, Scott M (1961) The effects of acetylcholine on the volume and vascular resistance of the dog's spleen. J Physiol 156:246–259
Edvinsson L, Emson P, McCulloch J, Tatemoto K, Uddman R (1983) Neuropeptide Y. Cerebrovascular innervation and vasomotor effects in the cat. Neurosci Lett 43:79–84
Fahrenkrug J, Schaffalitzky de Muckadell DB (1977) Radioimmunoassay of vasoactive intestinal polypeptide (VIP) in plasma. J Lab Clin Med 89:1379–1388
Fillenz M (1970) The innervation of the cat spleen. Proc R Soc Lond B 174:459–468
Gaddis RR, Dixon WR (1982) Presynaptic opiate receptor-mediated inhibition of endogenous norepinephrine and dopamine-β-hydroxylase release in the cat spleen, independent of presynaptic alpha-adrenoceptors. J Pharmacol Exp Ther 223:77–83
Gillespie JS, Kirpekar SM (1966) The histological localization of noradrenaline in the cat spleen. J Physiol 187:69–79
Goldstein M, Anagnoste B, Freedman LS, Roffman M, Ebstein RP, Park DH, Fuxe K, Hökfelt T (1973) Characterization. Localization and regulation of catecholamine synthesizing enzymes. In: Usdin E, Snyder SH (eds) Frontiers in catecholamine research, New York, Pergamon Press Inc, pp 69–81
Green HD, Ottis K, Kitchen T (1960) Autonomic stimulation and blockade on canine splenic inflow, outflow and weight. Am J Physiol 198:424–428
Hökfelt T, Fuxe K, Goldstein M, TH (1973) Immunohistochemical localization of three catecholamine-synthesizing enzymes: aspects on methodology. Histochemie 33:231–254
Järhult J, Hellstrand P, Sundler F (1980) Immunohistochemical localization and vascular effects of vasoactive intestinal polypeptide in skeletal muscle of the cat. Cell Tissue Res 207:55–64
Lembeck F, Holzer P (1979) Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn-Schmiedeberg's Arch Pharmacol 310:175–183
Lundberg JM, Tatemoto K (1982) Pancreatic polypeptide family (APP, BPP, NPY and PYY) in relation to sympathetic vasoconstriction resistant to α-adrenoceptor blockade. Acta Physiol Scand 116:393–402
Lundberg JM, Hökfelt T, Schultzberg M, Uvnäs-Wallensten K, Köhler C, Said SI (1979) Occurrence of vasoactive intestinal polypeptide (VIP)-like immunoreactivity in certain cholinergic neurons of the cat: Evidence from combined immunohistochemistry and acetylcholinesterase staining. Neuroscience 4:1539–1559
Lundberg JM, Hökfelt T, Änggård A, Terenius L, Elde R, Markey K, Goldstein M (1982a) Organization principles in the peripheral sympathetic nervous system: subdivisions by co-existing peptides (somatostatin, avian pancreatic polypeptide and vasoactive intestinal polypeptide-like immunoreactive materials. Proc Natl Acad Sci USA 79:1300–1307
Lundberg JM, Terenius L, Hökfelt T, Martling C-R, Tatemoto K, Mutt V, Polak J, Bloom S (1982b) Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand 116:477–480
Lundberg JM, Terenius L, Hökfelt T, Goldstein M (1983) High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett 42:167–172
Lundberg JM, Terenius L, Hökfelt T, Tatemoto K (1984) Comparative immunohistochemical and biochemical analysis of pancreatic polypeptide-like peptides with special reference to presence of neuropeptide Y in central and peripheral neurons. J Neurosci 4
Lundblad L, Änggård A, Lundberg JM (1984) Effects of antidromic trigeminal nerve stimulation in relation to parasympathetic vasodilation in cat nasal mucosa. Acta Physiol Scand 119:7–13
Malm L, Sundler F, Uddman R (1980) Effects of vasoactive intestinal polypeptide on resistance and capacitance vessels in the nasal mucosa. Acta Otolaryngol 90:304–308
Markey KA, Kondo S, Shenkman I, Goldstein M (1980) Purification and characterization of tyrosine hydroxylase from a clonal phaeochromocytoma cell line. Mol Pharmacol 17:79–85
Pearse AG, Polak JM (1975) Bifunctional reagents as vapour and liquid phase fixatives for immunohistochemistry. Histochem J 7:179–186
Peart WS (1949) The nature of splenic sympathin. J Physiol 108:491–501
Rehn NO (1958) Effect of decentralization on the content of catecholamines in the spleen and kidney of the cat. Acta Physiol Scand 42:309–312
Schultzberg M, Lundberg JM, Hökfelt T, Terenius L, Brand J, Elde R, Goldstein M (1978) Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience 3:1169–1186
Schultzberg M, Hökfelt T, Terenius L, Elfvin L-G, Lundberg JM, Brandt J, Elde RP, Goldstein M (1979) Enkephalin immunoreactive nerve fibres and cell bodies in sympathetic ganglia of the guinea-pig and rat. Neuroscience 4:249–270
Takasaki K, Tang L, Masanobu U (1979) The β-adrenergic responses in isolated splenic capsule area of several splecies of animals. Jpn J Pharmacol 29:1–7
Tatemoto K (1982) Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci USA 79:5485–5489
Tatemoto K, Carlqvist M, Mutt V (1982) Neuropeptide Y — a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature (Lond) 296:659–660
Utterback RA (1944) The innervation of the spleen. J Comp Neurol 81:55–67
Wilson SP, Klein RL, Chang U-J, Gasparis MS, Viveras OH, Yang WH (1980) Are opioid peptides co-transmitters in noradrenergic vesicles of sympathetic nerves? Nature 288:707–709
Yau WM, Youther ML (1982) Direct evidence for a release of acetylcholine from the myenteric plexus of guinea-pig small intestine by substance P. Eur J Pharmacol 81:655–668
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lundberg, J.M., Änggård, A., Pernow, J. et al. Neuropeptide Y-, substance P- and VIP-immunoreactive nerves in cat spleen in relation to autonomic vascular and volume control. Cell Tissue Res. 239, 9–18 (1985). https://doi.org/10.1007/BF00214896
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214896