Summary
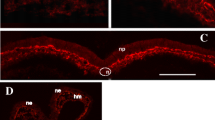
The initial migration of neural crest (NC) cells into cell-free space was studied by transmission electron microscopy at trunk levels of fowl embryos, some of which were fixed in the presence of ruthenium red. Migrating NC cells occurred in zones which contained fewer ruthenium-red stained 15–40 nm diameter granules than other regions. The ruthenium-red stained granules were linked by similarly stained thin (⪖ 3 nm diameter) microfibrils. The granules resemble proteoglycan and the microfibrils may be hyaluronate. NC cells contacted thicker (⪖ 10 nm diameter) fibrils and interstitial bodies, which did not require ruthenium red for visualization. Cytoplasmic microfilaments were sometimes aligned at the point of contact with the extracellular fibrils, which may be fibronectin and collagen.
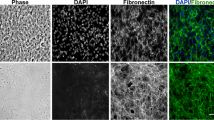
Phase-contrast time-lapse videotaping and scanning electron microscopy showed that NC cells of the fowl embryo in vitro migrated earlier and more extensively on glass coated with fibronectin-rich fibrous material and adsorbed fibronectin molecules than on glass coated with collagen type I (fibres and adsorbed molecules). NC cells became completely enmeshed in fibronectin-rich fibres, but generally remained on the surface of collagen-fibre gels. When given a choice, NC cells strongly preferred fibronectin coatings to plain glass, and plain glass to dried collagen gels. NC cells showed a slight preference for plain glass over glass to which collagen was adsorbed. Addition to the culture medium of hyaluronate (initial conc. 20 mg/ml), chondroitin (5 mg/ml) and fully sulphated chondroitin sulphate and dermatan sulphate (up to 10 mg/ml) did not drastically alter NC cell migration on fibronectin-rich fibrous substrates. However, partially desulphated chondroitin sulphate (5mg/ml) strongly retarded the migration of NC cells.
The in vivo and in vitro studies suggest that fibronectin may dictate the pathways of NC cell migration by acting as a highly preferred physical substrate. However, the utilization of these pathways may be reduced by the presence of proteoglycans bearing undersulphated chondroitin sulphate.
Similar content being viewed by others
Abbreviations
- NC :
-
neural crest
- ECM :
-
extracellular material
- GAG :
-
glycosaminoglycan
- FN :
-
fibronectin
- CIG :
-
cold insoluble globulin
- TEM :
-
transmission electron microscopy
- SEM :
-
scanning electron microscopy
- DMEM-H HEPES :
-
buffered Dulbecco's modified Eagle's medium
- FCS :
-
foetal calf serum
- CEE :
-
chick embryo extract
- SDS-PAGE :
-
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- PBS :
-
phosphate-buffered saline
References
Abercrombie M (1970) Contact inhibition in tissue culture. In vitro 6:128–142
Ali IU, Hynes RO (1978) Effects of LETS glycoprotein on cell motility. Cell 14:439–446
Alitalo K, Kurkinen M, Vaheri A, Krieg T, Timpl R (1980) Extracellular matrix components synthesized by human amniotic epithelial cells in culture. Cell 19:1053–1062
Bancroft M, Bellairs R (1976) The neural crest cells of the trunk region of the chick embryo studied by SEM and TEM. Zoon 4:73–85
Bard JBL, Hay ED, Meller SM (1975) Formation of the endothelium of the avian cornea: a study of cell movement in vivo. Dev Biol 42:334–361
Bolender DL, Seliger WG, Markwald RR (1980) A histochemical analysis of polyanionic compounds found in the extracellular matrix encountered by migrating cephalic neural crest cells. Anat Rec 196:401–412
Bornstein M (1958) Rat-tail collagen as a substrate. Lab Invest 7:134–137
Bruns RR, Gross J (1980) Collagen and glycosaminoglycans in cell adhesion. Exp Cell Res 128:1–7
Carlson E, Kenney MC (1980) Surface ultrastructure of the isolated avian notochord in vitro: the effect of the perinotochordal sheath. Anat Rec 197:257–276
Chen LB, Murray A, Segal RA, Bushnell A, Walsh ML (1978) Studies on intercellular LETS glycoprotein matrices. Cell 14:377–391
Chevallier A (1972) Localisation et durée des potentialitiés médulla-surrénaliennes des crêtes neurales chez l'embryon de poulet. J Embryol Exp Morphol 27:603–614
Cohen AM, Konigsberg IR (1975) A clonal approach to the problem of neural crest determination. Dev Biol 46:262–280
Comper WD, Laurent TC (1978) Physiological function of connective tissue polysaccharides. Physiol Rev 58:255–315
Davis EM (1980) Translocation of neural crest cells within a hydrated collagen lattice. J Embryol Exp Morphol 55:17–31
Davis EM, Trinkaus JP (1981) Significance of cell-to-cell contacts for the directional movement of neural crest cells within a hydrated collagen lattice. J Embryol Exp Morphol 63:29–51
Derby MA (1978) Analysis of glycosaminoglycans within the extracellular environments encountered by migrating neural crest cells. Dev Biol 66:321–336
Derby MA, Pintar JE (1978) The histochemical specificity of Streptomyces hyaluronidase and chondroitinase ABC. Histochem J 10:529–547
Douarin NM Le (1977) The differentiation of the ganglioblasts of the autonomic nervous system studied in chimeric avian embryos. In: Karkinen-Jääskeläinen M, SaxenLS, Weiss L (eds). Cell interactions in differentiation. Academic Press, London, pp 171–190
Ebendal T (1974) Scanning electron microscopy of chick embryo nerve fibres and heart fibroblasts on collagen substrata in vitro. Zoon 2:99–104
Ebendal T (1977) Extracellular matrix fibrils and cell contacts in the chick embryo. Possible roles in orientation of cell migration and axon extension. Cell Tissue Res 175:439–458
Elsdale T, Bard J (1972) Collagen substrate for studies on cell behaviour. J Cell Biol 54:626–637
Engvall E, Ruoslahti E (1977) Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer 20:1–5
Erickson CA, Tosney KW, Weston JA (1980) Invasiveness of neural crest and fibroblastic cells in embryonic tissues. Dev Biol 77:142–156
Fisher M, Solursh M (1979) The influence of the substratum on mesenchyme spreading in vitro. Exp Cell Res 123:1–14
Folger R, Weiss L, Glaves D, Subjeck JR, Harlos JP (1978) Translational movements of macrophages through media of different viscosities. J Cell Sci 31:245–257
Forrester JV, Wilkinson PC (1981) Inhibition of leukocyte locomotion by hyaluronic acid. J Cell Biol 48:315–331
Frederickson RG, Low FN (1971) The fine structure of perinotochordal microfibrils in control and enzyme-treated chick embryos. Am J Anat 130:347–376
Grinnell F, Feld MK (1979) Initial adhesion of human fibroblasts in serum-free medium: possible role of secreted fibronectin. Cell 17:118–130
Grinnell F, Minter D (1978) Attachement and spreading of baby hamster kidney cells to collagen substrata: Effects of cold-insoluble globulin. Proc Natl Acad Sci USA 75:4408–4412
Harris A (1973) Behavior of cultured cells on substrata of variable adhesiveness. Exp Cell Res 77:285–297
Harris AK (1974) Contact inhibition of cell locomotion. In: Cox RP (ed) Cell communication. John Wiley and Sons, New York London, pp 147–185
Hay ED (1978) Fine structure of embryonic matrices and their relation to the cell surface in ruthenium red-fixed tissue. Growth 42:399–423
Heaysman JEM (1978) Contact inhibition of locomotion: a reappraisal. Int Rev Cytol 55:49–66
Hedman K, Vaheri A, Wartiovaara J (1978) External fibronectin of cultured human fibroblasts is predominantly a matrix protein. J Cell Biol 76:748–760
Hedman K, Kurkinen M, Alitalo K, Vaheri A, Johansson S, Höök M (1979) Isolation of the pericellular matrix of human fibroblast cultures. J Cell Biol 81:83–91
Holmdahl DE (1928) Die Entstehung und weitere Entwicklung der Neuralleiste (Ganglienleiste) bei Vögeln und Säugetieren. Z Mikrok — anat Forsch 14:99–298
Hsieh P, Segal R, Chen LB (1980) Studies of fibronectin matrices in living cells with fluoresceinated gelatin. J Cell Biol 87:14–22
Johnson LD, Warfel J, Megaw JM (1978) Synthesis of an epithelial basement membrane glycoprotein by cultured human and murine cells. In: Kefalides NA (ed) Biology and chemistry of basement membranes. Academic Press, London, pp 299–308
JohnstonMC (1966) A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat Rec 156:143–156
Kanwar YS, Farquhar MG (1979) Isolation of glycosaminoglycans (heparan sulphate) from glomerular basement membranes. Proc Natl Acad Sci USA 76:4493–4497
Kefalides NA (1978) Current status of chemistry and structure of basement membranes. In: Kefalides NA (ed) Biology and chemistry of basement membranes. Academic Press, London, pp 215–228
KenneyMC, Carlson E (1978) Ultrastructural identification of collagen and glycosaminoglycans in notochordal extracellular matrix in vivo and in vitro. Anat Res 190:827–850
Knox P, Wells P (1979) Cell adhesion and proteoglycans. I. The effect of exogenous proteolgycans on the attachment of chick embryo fibroblasts to tissue culture plastic and collagen. J Cell Sci 40:77–88
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laterra J, Ansbacher R, Culp LA (1980) Glycosaminoglycans that bind cold-insoluble globulin in cell-substratum adhesion sites of murine fibroblasts. Proc Natl Acad Sci USA 77:6662–6666
Leivo I, Vaheri A, Timpl R, Wartiovaara J (1980) Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol 76:100–114
Linder E, Vaheri A, Ruoslahti E, Wartiovaara J (1975) Distribution of fibroblast surface antigen in the developing chick embryo. J Exp Med 142:41–49
Löfberg J, Ahlfors K, Fällström C (1980) Neural crest cell migration in relation to extracellular matrix organization in the embryonic axolotl trunk. Dev Biol 75:148–167
Low FN (1970) Interstitial bodies in the early chick embryo. Am J Anat 128:45–56
Luft JH (1971) Ruthenium red and violet. II Fine structural localization in animal tissues. Anat Rec 171:369–416
Lunt DM, Seegmiller RE (1980) Differential localization of 35S sulfate within ectodermal basement membrane in relation to initiation of chick limb buds. J Embryol Exp Morphol 60:189–199
Madri JA, Roll FJ, Furthmayr H, Foidart J-M (1980) Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol 86:682–687
Manasek FJ (1975) The extracellular matrix: a dynamic component of the developing embryo. Curr Top Dev Biol 10:35–102
Mark H von der, Mark K von der, Gay S (1976) Study of differential collagen synthesis during development of the chick embryo by immunofluorescence: I. Preparation of collagen type I and type II specific antibodies and their application to early stages of the chick embryo. Dev Biol 48:237–249
Markwald RR, Fitzharris TP, Bolender DL, Bernanke DH (1979) Structural analysis of cell: matrix association during morphogenesis of atrioventricular cushion tissue. Dev Biol 69:634–654
Maxwell GD (1976) Substrate dependence of cell migration from explanted neural tubes in vitro. Cell Tissue Res 172:325–330
Mayer BW, Packard DS (1978) A study of the expansion of the chick area vasculosa. Dev Biol 63:335–351
Mayer BW jr, Hay ED, Hynes RO (1981) Immunocytochemical localization of fibronectin in embryonic chick trunk and area vasculosa. Dev Biol 82:267–286
Minor RR (1973) Somite chondrogenesis. A structural analysis. J Cell Biol 56:27–50
Nagasawa K, Inoue Y, Tokuyasu T (1979) An improved method for the preparation of chondroitin by solvolytic desulfation of chondroitin sulfates. J Biochem 86:1323–1329
Newgreen DF, Gibbins IL (1981) Factors controlling the time of onset of neural crest cell migration in the Fowl embryo. Submitted to Cell Tissue Res
Newgreen D, Thiery J-P (1980) Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res 211:269–291
Newgreen DF, Ritterman M, Peters EA (1979) Morphology and behaviour of neural crest cells of chick embryo in vitro. Cell Tissue Res 203:115–140
Noden D (1975) An analysis of the migratory behaviour of avian cephalic neural crest cells. Dev Biol 42:106–130
Noden D (1978) Interactions directing the migration and cytodifferentiation of avian neural crest cells. In: Garrod DR (ed) Specificity of embryological interactions. Receptors and recognition. Series B Vol 4. Chapman and Hall, London, pp 5–49
OvertonJ (1979) Differential response of embryonic cells to culture on tissue matrices Tissue Cell 11:89–98
Paul J (1970) Cell and tissue culture. 4th edition. Livingstone, Edinburgh and London
Pintar J (1978) Distribution and synthesis of glycosaminoglycans during quail neural crest morphogenesis. Dev Biol 67:444–464
Pratt RM, Larsen MA, Johnston MC (1975) Migration of cranial neural crest cells in a cell-free hyaluronate-rich matrix. Dev Biol 44:298–305
Rodén L (1980) Structure and metabolism of connective tissue proteoglycans. In: LennarzWJ (ed) The biochemistry of glycoproteins and proteoglycans. Plenum Press, New York and London, pp 267–371
RoseG (1954) A separable multipurpose tissue culture chamber. Tex Rep Biol Med 12:1074–1083
Rose GG, Pomerat CM, Shindler TO, Trunnel JB (1958) A cellophane-strip technique for culturing tissue in multipurpose culture chambers. J Biophys Biochem Cytol 4:761–764
Rubin H (1973) Chick embryo cells. In: Kruse PF Jr, Patterson MK Jr (eds) Tissue culture: methods and applications. Academic Press, London, pp 119–22
Sanders EJ (1980) The effect of fibronectin and substratum attached material on the spreading of chick embryo mesoderm cells in vitro. J Cell Sci 44:225–242
Schor SL, Schor AM, Bazill GW (1981) The effect of fibronectin on the migration of human foreskin fibroblasts and Syrian hamster melanoma cells into three-dimensional gels of native collagen fibres. J Cell Sci 48:301–314
Singer II (1978) The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 16:675–685
Singley CT, Solursh M (1980) The use of tannic acid for the ultrastructural visualization of hyaluronic acid. Histochem. 65:93–102
Solursh M, Fisher M, Meier S, Singley CT (1979) The role of extracellular matrix in the formation of the sclerotome. J Embryol Exp Morphol 54:75–98
Teillet MA, Douarin NM Le (1970) La migration des cellules pigmentaires étudiée par la méthode des greffes hétérospécifiques de tube nerveux chez l'embryon d'Oiseau. C R Acad Sci Ser D 270:3095–3098
Toole BP (1976) Morphogenetic role of glycosaminoglycans (acid mucopolysaccharides) in brain and other tissues. In: Barondes SH (ed) Neuronal recognition. Plenum Press, New York, pp 275–329
TosneyKW (1978) The early migration of neural crest cells in the trunk region of the avian embryo: an electron microscopic study. Dev Biol 62:317–333
Trelstad RL, Hayashi K, Toole BP (1974) Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol 62: 812–830
Venable JH, Coggeshall R (1965) A simplified lead citrate stain for use in electron microscopy. J Cell Biol 25:407–408
Weston JA (1963) A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev Biol 6:279–310
Weston JA (1970) The migration and differentiation of neural crest cells. Adv Morphogen 8:41–114
Weston JA, Butler SL (1966) Temporal factors affecting localization of neural crest cells in the chicken embryo. Dev Biol 14:246–266
Weston JA, Derby MA, Pintar JE (1978) Changes in the extracellular environment of neural crest cells during their early migration. Zoon 6:102–112
Wight TN, Ross R (1975) Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol 67:660–674
Yamada KM, Yamada SS, Pastan I (1976) Cell surface protein partially restores morphology, adhesiveness and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci USA 73:1217–1221
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Newgreen, D.F., Gibbins, I.L., Sauter, J. et al. Ultrastructural and tissue-culture studies on the role of fibronectin, collagen and glycosaminoglycans in the migration of neural crest cells in the fowl embryo. Cell Tissue Res. 221, 521–549 (1982). https://doi.org/10.1007/BF00215700
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00215700