Summary
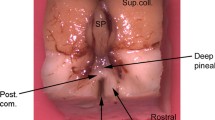
A combined thin-section/freeze-fracture study was performed on the superficial pineal gland of the golden hamster, comparing the parenchymal and interstitial cells of this animal with those previously investigated in rats. In contrast to rats, no gap junctions and gap/tight junction combinations could be found between pineal parenchymal cells of the hamster. Furthermore, the interstitial cells of the hamster pineal gland were found to have large flat cytoplasmic processes, which abut over large areas equipped with tight junctions. In thin sections, profiles of interstitial cell processes were seen to surround groups of pinealocytes. Interstitial cells and their sheet-like, tight junction-sealed processes thus appear to delimit lobule-like compartments of the hamster pineal gland. Because the classification of the interstitial cells is uncertain, the expression of several markers characteristic of mature and immature astrocytes and astrocyte subpopulations has been investigated by indirect immunohistology. Many of the non-neuronal elements in the pineal gland are vimentin-positive glial cells, subpopulations of which express glial fibrillary acidic protein (GFA) and C1 antigen. The astroglial character of these cells is supported by the lack of expression of markers for neuronal, meningeal and endothelial cells. M1 antigen-positive cells have not been detected.
Similar content being viewed by others
References
Anders JJ, Brightman MW (1979) Assemblies of particles in the cell membranes of developing, mature and reactive astrocytes. J Neurocytol 8:777–795
Bignami A, Dahl D (1974) Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to glial fibrillary acidic protein. J Comp Neurol 153:27–37
Bucana CD, Nadakavukaren MJ, Frehn JL (1971) Annulate lamellae in hamster pineal gland. Tissue & Cell 3:405–412
Clabough JW (1971) Ultrastructural features of the pineal gland in normal and light deprived golden hamsters. Z Zellforsch 114:151–164
Cullen MJ, Gulley RL (1980) Freeze-fracture studies of the macromolecular organization of glial membranes. TINS, May 1980, pp 113–116
Dimpfel W, Huang RTC, Habermann E (1977) Gangliosides in nervous tissue and binding of 125I-labelled tetanus toxin, a neuronal marker. J Neurochem 29:329–334
Eng LF, Vanderhaeghen JJ, Bignami A, Gerstl B (1971) An acidic protein isolated from fibrous astrocytes. Brain Res 28:351–354
Forssmann WG, Ito S, Weihe E, Aoki A, Dym M, Fawcett DW (1977) An improved perfusion method for the testis. Anat Rec 188:307–314
Franke WW, Schmid E, Osborn M, Weber K (1979) Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res 123:25–46
Goridis C, Martin J, Schachner M (1978) Characterization of an antiserum to synaptic glomeruli from rat cerebellum. Brain Res 148:269–278
Huang S-K, Taugner R (1983) Gap junctions between guinea pig pinealocytes. Cell Tissue Res (submitted for publication)
Lagenaur C, Sommer I, Schachner M (1980) Subclass of astroglia recognized in mouse cerebellum by monoclonal antibody. Dev Biol 79:367–378
Landis DMD, Reese TS (1974) Arrays of particles in freeze-fractured astrocytic membranes. J Cell Biol 60:316–320
Lin H-S, Hwang B-H, Tseng C-Y (1975) Fine structural changes in the hamster pineal gland after blinding and superior cervical ganglionectomy. Cell Tissue Res 158:285–299
Lindner J, Rathjen F, Schachner M (1983) L1 mono and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum, Nature (in press)
Löwenthal A, Flament-Durand J, Karcher D, Noppe M, Brion JP (1982) Glial cells identified by anti-α-albumin (anti-GFA) in human pineal gland. J Neurochem 38:863–865
Mirsky R, Wendon LMB, Black P, Stolkin C, Bray D (1978) Tetanus toxin: a cell surface marker for neurons in culture. Brain Res 148:251–259
Møller M, Ingild A, Bock E (1978) Immunohistochemical demonstration of S-100 protein and GFA protein in interstitial cells of the rat pineal gland. Brain Res 140:1–13
Rathjen FG, Schachner M (1983) Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion, EMBO J (in press)
Rueger DC, Huston JS, Dahl D, Bignami A (1979) Formation of 100 Å filaments from purified glial fibrillary acidic protein in vitro. J Mol Biol 135:53–68
Schachner M (1982) Glial antigens and the expression of neuroglial phenotypes. Trends Neurosci 5:225–228
Schachner M, Willinger M (1979) Cell type specific cell surface antigens in the cerebellum. Prog Brain Res 51:23–44
Schachner M, Hedley-Whyte T, Hsu D, Schoonmaker G, Bignami A (1977) Ultrastructural localization of glial fibrillary acidic protein in mouse cerebellum by immunoperoxidase labelling. J Cell Biol 75:67–73
Schachner M, Huang S-K, Ziegelmüller P, Bizzini B, Taugner R (1983) Glial cells in the pineal gland of mice and rats. A combined immunofluorescence and electron microscopic study. Cell Tissue Res (submitted for publication)
Schachner M, Schoonmaker G, Hynes RO (1978) Cellular and subcellular localization of LETS protein in the nervous system. Brain Res 158:149–158
Schnitzer J, Schachner M (1981) Expression of Thy-1, H-2 and NS-4 cell surface antigens and tetanus toxin receptors in early postnatal and adult mouse cerebellum. J Neuroimmunol 1:429–456
Schnitzer J, Franke WW, Schachner M (1981) Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol 90:435–475
Sheridan MN, Reiter RJ (1968) The fine structure of the hamster pineal gland. Am J Anat 122:357–376
Sommer I, Schachner M (1981) Expression of glial antigens C1 and M1 in developing and adult neurologically mutant mice. J Supramol Struct, Cell Biochem 16:53–74
Sommer I, Lagenaur C, Schachner M (1981) Recognition of Bergmann glial and ependymal cells in the mouse nervous system by monoclonal antibody. J Cell Biol 90:448–458
Taugner R, Schiller A, Rix E (1981) Gap junctions between pinealocytes. A freeze-fracture study of the pineal gland in rats. Cell Tissue Res 218:303–314
Vollrath L (1981) The pineal organ. In: Oksche A, Vollrath L (eds) Handbuch der mikroskopischen Anatomie des Menschen (VI/7). Springer, Berlin Heidelberg New York
Wolfe DE (1965) The epiphyseal cell: an electron-microscopic study of its intercellular relationships and intracellular morphology in the pineal body of the albino rat. In: Kappers JA, Schade JP (eds) Structure and function of the epiphysis cerebri. Progress in Brain Res, Vol 10, Elsevier Publishing Company, Amsterdam London New York, pp 332–376
Yen S-H, Fields KL (1981) Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol 88:115–126
Author information
Authors and Affiliations
Additional information
Supported by a grant from Deutsche Forschungsgemeinschaft (Scha 185/9-2)
Rights and permissions
About this article
Cite this article
Huang, S.K., Nobiling, R., Schachner, M. et al. Interstitial and parenchymal cells in the pineal gland of the golden hamster. Cell Tissue Res. 235, 327–337 (1984). https://doi.org/10.1007/BF00217857
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217857