Abstract
Dissociation of protein-containing structures by modification of protein amino groups with dicarboxylic acid anhydrides is a mild procedure which, in some cases, offers advantages over treatment with alternative dissociating agents, such as urea, guanidine hydrochloride, detergents, high ionic strength, and extremes of pH: In addition to dissociating multimeric proteins and protein aggregates, dicarboxylic acid anhydrides are effective dissociating agents for membrane-bound proteins and nucleoprotein particles. With most dicarboxylic acid anhydrides reviewed, the introduced reagent residues can be eliminated under moderate acid conditions, which allows the purification of unmodified individual components, and the use of disassembly-reconstitution systems valuable for investigating the structural and functional roles played by the individual components of complex particles:
Each reagent can be suitable for a particular purpose, depending on the required specificity of the modification and stability of the modified groups: The stability of the acylated amino groups ranges from the very stable succinylated amino groups to the very labile acylation obtained with dimethylmaleic anhydride: Between these extremes, the stability of the modified amino groups decreases stepwise in the following order: maleic, exo-cis-3,6-endoxo-Δ4-tetrahydrophthalic, citraconic, and 3,4,5,6-tetrahydrophthalic anhydride. With respect to the selectivity of the produced modification, little or no modification of hydroxyamino acid and cysteine residues has been observed with dimethylmaleic, exo-cis-3,6-endoxo-Δ4-tetrahydrophthalic, and 3,4,5,6-tetrahydrophthalic anhydrides: With the other reagents, the extent of modification of hydroxyamino acid residues increases in the order citraconic, maleic and succinic anhydride: Citraconic and maleic anhydrides can produce irreversible modification of cysteine residues, the reactivity of sulfhydryl groups being higher with maleic anhydride:
Similar content being viewed by others
References
Means GE, Feeney RE: Chemical modification of proteins. Holden-Day, San Francisco, 1971
Klotz IM, Keresztes-Nagy S: Dissociation of proteins into subunits by succinylation: Haemerythrin. Nature 195: 900–901, 1962
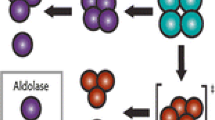
Hass LF: Aldolase dissociation into subunits by reaction with succinic anhydride. Biochemistry 3: 535–541, 1964
Polyanovsky OL: Reversible dissociation of succinylated aspartate transaminase into subunits. Biochem Biophys Res Commun 19: 364–370, 1965
Freisheim JH, Walsh KA, Neurath H: The activation of bovine procarboxypeptidase A. I: Isolation and properties of the succinylated enzyme precursor. Biochemistry 6: 3010–3019, 1967
Sia CL, Horecker BL: Dissociation of protein subunits by maleylation. Biochem Biophys Res Commun 31: 731–737, 1968
Gibbons I, Perham RN: The reaction of aldolase with 2methylmaleic anhydride. Biochem J 116: 843–849, 1970
Puigserver A, Desnuelle P: Dissociation of bovine 6S procarboxypeptidase A by reversible condensation with 2,3dimethyl maleic anhydride: Application to the partial characterization of subunit III. Proc Nat Acad Sci USA 72: 2442–2445, 1975
Butler PJG, Hartley BS: Maleylation of amino groups. Methods Enzymol 25: 191–199, 1972
Klapper MH, Klotz IM: Acylation with dicarboxylic acid anhydrides. Methods Enzymol 25: 531–536, 1972
Lundahl P: Proteins selectively released from water-extracted human erythrocyte membrane upon citraconylation or maleylation. Electrophoretic analysis and chromatographic fractionation. Biochim Biophys Acta 379: 304–316, 1975
Howlett GJ, Wardrop AJ: Dissociation and reconstitution of human erythrocyte membrane proteins using 3,4,5,6-tetrahydrophthalic anhydride. Arch Biochem Biophys 188: 429–437, 1978
Pintor-Toro JA, Vázquez D, Palacian E: Reversible modification of Escherichia coli ribosomes with 2,3-dimethylmaleic anhydride: A new method to obtain protein-deficient ribosomal particles. Biochemistry 18: 3219–3223, 1979
T'sai AL, Palmer G: Purification and characterization of highly purified cytochrome b from complex III of baker's yeast. Biochim Biophys Acta 681: 484–495, 1982
Jordano J, Montero F, Palacian E: Rearrangement of nucleosomal components by modification of histone amino groups: Structural role of lysine residues. Biochemistry 23: 4280–4284, 1984
Butler PJG, Harris JI, Hartley BS, Leberman R: The use of maleic anhydride for the reversible blocking of amino groups in polypeptide chains. Biochem J 112: 679–689, 1969
Butler PJG, Harris JI, Hartley BS, Leberman R: Reversible blocking of peptide amino groups by maleic anhydride. Biochem J 103: 78P-79P, 1967
Dixon HBF, Perham RN: Reversible blocking of amino groups with citraconic anhydride. Biochem J 109: 312–314, 1968
Kirby AJ, Lancaster PW: Structure and efficiency in intramolecular and enzymic catalysis: Catalysis of amide hydrolysis by the carboxy-group of substituted maleamic acids. J Chem Soc Perkin Trans II 1206–1214, 1972
Nieto MA, Palacidn E: Effects of temperature and pH on the regeneration of the amino groups of ovalbumin after modification with citraconic and dimethylmaleic anhydrides. Biochim Biophys Acta 749: 204–210, 1983
Habeeb AFSA, Cassidy HG, Singer SJ: Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta 29: 587–593, 1958
Riordan JF, Vallee BL: Succinylcarboxypeptidase. Biochemistry 3: 1768–1774, 1964
Gounaris AD, Perlmann GE: Succinylation of pepsinogen. J Biol Chem 242: 2739–2745, 1967
King L, Perham RN: Reaction of tobacco mosaic virus with maleic anhydride and some possible applications to X-ray diffraction analysis. Biochemistry 10: 981–987, 1971
Bernad A, Nieto MA, Vioque A, Palacian E: Modification of the amino and hydroxyl groups of lysozyme with carboxylic acid anhydrides: A comparative study. Biochim Biophys Acta 873: 350–355, 1986
Brinegar AC, Kinsella JE: Reversible modification of lysine in β-lactoglobulin using citraconic anhydride: Effects on the sulfhydryl groups. Int J Peptide Protein Res 18: 18–25, 1981
De la Escalera S, Palacian E: Dimethylmaleic anhydride, a specific reagent for protein amino groups. Biochem Cell Biol 67: 63–66, 1989
Riley M, Perham RN: The reversible reaction of protein amino groups with exo-cis-3,6-endoxo-Δ4-tetrahydrophthalic anhydride. The reaction with lysozyme. Biochem J 118: 733–739, 1970
Gibbons I, Schachman HK: A method for the separation of hybrids of chromatographically identical oligomeric proteins. Use of 3,4,5,6-tetrahydrophthaloyl groups as a reversible ‘chromatographic handle’. Biochemistry 15: 52–60, 1976
Klotz IM, Keresztes-Nagy S: Hemerythrin: Molecular weight and dissociation into subunits. Biochemistry 2: 445–452, 1963
Jaenicke R, Schmidt D, Knof S: Monodispersity and quaternary structure of glyceraldehyde 3-phosphate dehydrogenase. Biochemistry 7: 919–926, 1968
Marmocchi F, Mavelli I, Rigo A, Stevanato R, Bossa F, Rotilio G: Succinylated copper, zinc superoxide dismutase. A novel approach to the problem of active subunits. Biochemistry 21: 2853–2856, 1982
Bindels JG, Misdom LW, Hoenders HJ: The reaction of citraconic anhydride with bovine α-crystallin lysine residues. Surface probing and dissociation-reassociation studies. Biochim Biophys Acta 828: 255–260, 1985
MacLennan DH, Tzagoloff A, Rieske JS: Studies on the electron transfer system: LXIII. Solubilization and fractionation of mitochondrial proteins by succinylation. Arch Biochem Biophys 109: 383–387, 1965
Moldow CF, Zucker-Franklin D, Gordon A, Hospelhorn V, Silber R: Studies on the succinylation of erythrocyte membranes. Biochim Biophys Acta 255: 133–148, 1972
Eshhar Z, Gafni M, Givol D, Sela M: Solubilization of lymphocyte and thymocyte antigens by a reversible chemical modification. Eur J Immunol 1: 323–329, 1971
Bustin M, Eshhar Z, Sela M: Characterization and partial purification of antigenic components solubilized by a reversible chemical modification from rat-thymocyte membrane. Eur J Biochem 31: 541–553, 1972
Vadlamudi BP, Dzandu JD, Larway PF: Differential solubilization of human erythrocyte cell membrane proteins by maleic anhydride. Biochem Biophys Res Commun 64: 64–71, 1975
Palacián E: Solubilization of membrane-bound transpeptidase from Streptomyces strain K-11 with 2,3-dimethylmaleic anhydride. Rev Esp Fisiol 35: 481–484, 1979
Stuart A, Khorana HG: The selective acetylation of terminal hydroxyl groups in deoxyribooligonucleotides. J Am Chem Soc 85: 2346–2347, 1963
Stuart A, Khorana HG: Studies on polynucleotides. XXXIII. The labeling of end groups in polynucleotide chains: the selective acetylation of terminal hydroxyl groups in deoxyribopolynucleotides. J Biol Chem 239: 3885–3892, 1964
Vioque A, Hernández F, Palacián E: Effects of different amino-group reagents on ribosomal integrity: Structural role of lysine residues. Molec Biol Rep 11: 47–50, 1986
Shetty JK, Kinsella JE: Ready separation of proteins from nucleoprotein complexes by reversible modification of lysine residues. Biochem J 191: 269–272, 1980
Shetty JK, Kinsella JE: Reversible modification of lysine: separation of proteins and nucleic acids in yeast. In: RE Feeney, JR Whitaker (eds) Modification of Proteins (Food, Nutritional, and Pharmacological Aspects). Advances in Chemistry Series 198, American Chemical Society, Washington DC, 1982, pp 169–198
Palacián E, López-Rivas A, Pintor-Toro JA, Hernández F: Dissociation of the protein components from chromatin by reversible modification with dimethylmaleic anhydride. Molec Cell Biochem 36: 163–167, 1981
Jordano J, Montero F, Palacián E: Relaxation of chromatin structure upon removal of histones H2A and H2B. FEBS Lett 172: 70–74, 1984
Nieto MA, Palacián E: Pitfalls in the use of carboxylic acid anhydrides for structural studies of nucleoprotein particles. Biochem J 241: 621–623, 1987
Nieto MA, Palacián E: Structural changes of nucleosomal particles and isolated core-histone octamers induced by chemical modification of lysine residues. Biochemistry 27: 5635–5640, 1988
Jordano J, Nieto MA, Palacián E: Dissociation of nucleosomal particles by chemical modification: Equivalence of the two binding sites for H2A · H2B dimers. J Biol Chem 260: 9382–9384, 1985
De la Escalera S, Nieto MA, Palacián E: Preparation and structural characterization of nucleosomal core particles lacking one H2A · H2B dimer. Biochem Biophys Res Commun 157: 541–547, 1988
González PJ, Martínez C, Palacián E: Interaction with RNA polymerase of nucleosomal cores lacking one H2A H2B dimer. J Biol Chem 262: 11280–11283, 1987
González PJ, Palacián E: Interaction of RNA polymerase II with structurally altered nucleosomal particles. Transcription is facilitated by loss of one H2A · H2B dimer. J Biol Chem 264: 18457–18462, 1989
Baer BW, Rhodes D: Eukaryotic RNA polymerase II binds to nucleosome cores from transcribed genes. Nature 301: 482–488, 1983
Jackson V: Deposition of newly synthesized histones: new histones H2A and H2B do not deposit in the same nucleosome with new histones H3 and H4. Biochemistry 26: 2315–2325, 1987
Jackson V: Deposition of newly synthesized histones: hybrid nucleosomes are not tandemly arranged on daughter DNA strands. Biochemistry 27: 2109–2120, 1988
Pintor-Toro JA, Vázquez D, Palacián E: Effects on ribosomal activity and structure of modification with succinic, maleic and acetic anhydrides. FEBS Lett 87: 125–128, 1978
Vioque A, Pintor-Toro JA, Palacián E: Partial reconstitution of active eukaryotic ribosomes following dissociation with dimethylmaleic anhydride. J Biol Chem 257: 6477–6480, 1982
Pintor-Toro JA, Hernández F, López-Rivas A, Palacián E: Reversible modification of 50 S ribosomal subunits with dimethylmaleic anhydride. Protein-deficient particles. Molec Cell Biochem 43: 43–47, 1982
Hernandez F, Vioque A: Modification of 40 S ribosomal subunits from yeast with dimethylmaleic anhydride. Molec Biol Rep 11: 137–141, 1986
Conquer F, Lavergne JP, Paleologue A, Reboud JP, Rebond AM: Partial reassembly of active 60 S ribosomal subunits from rat liver following treatment with dimethylmaleic anhydride. Eur J Biochem 163: 15–20, 1987
Garrido S, González PJ, Palacián E, Hernández F: Reconstitution of rat liver 60 S ribosomal subunits following disassembly by dimethylmaleic anhydride. Molec Cell Biochem 92: 159–167, 1990
Pintor-Toro JA, López-Rivas A, Hernández F, Palacián E: Dissociation of proteins from Escherichia coli ribosomes after dimethylmaleic anhydride treatment. Effects of elongation factor G and antibiotics. FEBS Lett 135: 21–24, 1981
Vioque A, Palacián E: Protein-deficient ribosomal particles from yeast 60 S subunits obtained by modification with dimethylmaleic anhydride and by treatment with NH4Cl. Rev Esp Fisiol 41: 287–292, 1985
Nieto MA, Hernández F, Palacián E: Disassembly and reconstitution of yeast 60 S ribosomal subunits. Molec Cell Biochem 86: 55–63, 1989
Vioque A, Hernández F, Palacián E: Involvement of lysine and arginine residues in the binding of yeast ribosomal protein YL3 to 5S RNA. Molec Cell Biochem 76: 141–146, 1987
Pintor-Toro HJ, Hernández F, López-Rivas A, Palacián E: Protein-deficient ribosomal particles obtained by reversible modification with dimethylmaleic anhydride. Arch Biochem Biophys 210: 786–789, 1981
Vioque A, Palacián E: Partial reconstitution of 60 S ribosomal subunits from yeast. Molec Cell Biochem 66: 55–60, 1985
Bielka H, Stahl J, Bommer UA, Welfle H, Noll F, Westerman P: Interaction, topography and function of ribosomal components. In: H Bielka (ed) The Eukaryotic Ribosome. Springer-Verlag, Berlin, 1982, pp 162–185
Lee JC, Anderson R: Partial reassembly of yeast 60 S ribosomal subunits in vitro following controlled dissociation under nondenaturing conditions. Arch Biochem Biophys 245: 248–253, 1986
Brinegar AC, Kinsella JE: Reversible modification of lysine in soybean proteins, using citraconic anhydride: characterization of physical and chemical changes in soy protein isolate, the 7S globulin, and lipoxygenase. J Agric Food Chem 28: 818–824, 1980
Habeeb AFSA, Atassi MZ: Enzymic and immunochemical properties of lysozyme. Evaluation of several amino group reversible blocking reagents. Biochemistry 9: 4939–4944, 1970
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Palacián, E., González, P.J., Piñeiro, M. et al. Dicarboxylic acid anhydrides as dissociating agents of protein-containing structures. Mol Cell Biochem 97, 101–111 (1990). https://doi.org/10.1007/BF00221051
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00221051