Abstract
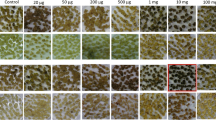
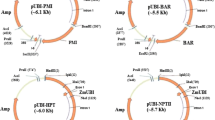
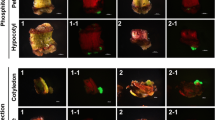
Phosphinothricin (PPT) inhibits glutamine synthetase in plant cells, resulting in an accumulation of ammonia that can be toxic. Bar, from Streptomyces hygroscopicus is a gene that codes for phosphinothricin acetyl-transferase, an enzyme that detoxifies PPT by acetylation. We used polyethylene-glycol-mediated transformation to introduce bar into protoplasts of maize (Zea mays L.). With a novel assay utilizing the pH indicator chlorophenol red we identified transformants after only two to four weeks of selection on PPT.
Similar content being viewed by others
Abbreviations
- BT:
-
Bacillus thuringiensis
- CR assay:
-
chloro-phenol-red assay
- EDTA:
-
(ethylenedinitro)tetraacetic acid disodium salt
- PAT:
-
phosphinothricin acetyltransferase
- PCR:
-
polymerase chain reaction
- PEG:
-
polyethylene glycol
- PPT:
-
phosphinothricin
- TRIS:
-
(hydroxymethyl)aminomethane
References
Botterman, J., Leemans, J. (1989) Discovery, transfer to crops, expression and biological significance of a Bialaphos resistance gene. 1989 BCPC Mono. No. 42 Amino acid biosynthesis inhibitors, pp. 63–68
Carswell, G.K., Johnson, C.M., Shillito, R.D., Harms, C.T. (1989) O-acetyl-salicylic acid promotes colony formation from protoplasts of an elite maize inbred. Plant Cell Rep. 8, 282–284
Chu, C.C., Wang, C.C., Sun, C.S., Hsu, C., Yin, K.G., Chu, C.Y., Bi, F.Y. (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci. Sin. 18, 659–668
De Block, M., Botterman, J. Vandewiele, M. Dockx, J. Thoen, C. Gosselé, V. Rao Movva, N. Thompson, C. Van Montague, M., Leemans, J. (1987) Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 6, 2513–2518
De Block, M., De Brouwer, D., Tenning, P. (1989) Transformation of Brassica napus and Brassica oleracea using Aprobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol. 91, 694–701
Dekeyser, R., Claes, B., Marichal, M., Montagu, M.V., Caplan, A. (1989) Evaluation of selectable markers for rice transformation. Plant Physiol, 90, 217–223
DiMaio, J.J., Shillito R.D. (1989) Cryopreservation technology for plant cell cultures. J. Tissue Cult. Methods 12, 163–169
Gordon-Kamm, W.J., Spencer, M.T., Mangano, M.L., Adams, T.R., Daines, R.J., Start, W.G., O'Brien, J.V., Chambers, S.A., Adams, W.R., Willets, N.G., Rice, T.B., Mackey, C.J., Krueger, R.W., Kausch, A.P., Lemaux, P.G. (1990) Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2, 603–618
Johnson, C.M., Carswell. G.K., Shillito, R.D. (1989) Direct gene transfer via polyethylene glycol. J. Tissue Cult. Methods 12, 127–133
Kao, K.N., Michayluk, M.R. (1975) Nutrient requirements for growth of Vicia hajastana cells and protoplasts at very low population density in liquid media. Planta 126, 105–110
Koziel, M.G., Beland, G.L., Bowman, C., Carozzi, N.B., Cren-shaw R., Crossland, L., Dawson, J., Desai, N., Hill, M., Kad-well, S., Launis, K., Lewis, K., Maddox, D., McPherson, K., Meghji, M.R., Merlin, E., Rhodes, R., Warren, G.W., Wright, M., Evola, S.V., (1993) Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Bio/technology 11, 194–200
Krieg, L.C., Walker, M.A., Senaratna, T., McKersie, B.D. (1990) Growth, ammonia accumulation and glutamine synthetase activity in alfalfa (Medicago sativa L.) shoots and cell cultures treated with phosphinothricin. Plant Cell Rep. 9, 80–83
Negrutiu, I., Shillito, R., Potrykus, L, Biasini, G., Sala, F. (1987) Hybrid genes in the analysis of transformation conditions. Plant Mol. Biol 8, 363–373
Paszkowski, J., Shillito, R.D., Saul, M., Mandak, V., Hohn, T., Hohn, B., Potrykus, I. (1984) Direct gene transfer to plants. EMBO J. 3, 2717–2722
Ryu, D.D.Y., Lee, S.O., Romani, R.J. (1990) Determination of growth rate for plant cell cultures: comparative studies. Biotech. Bioeng. 35, 305–311
Shang, X.M., Huang, J.Y., Haigler, C.H., Trolinder, N.L. (1991) Buffer capacity of cotton cells and effects of extracellular pH on growth and somatic embryogenesis in cotton cell suspensions. In Vitro Cell Dev. Biol. 27P, 147–152
Shillito, R.D., Carswell. G.K., Johnson, C.M., DiMaio, J.J., Harms, C.T. (1989) Regeneration of fertile plants from protoplasts of elite inbred maize. Bio/technology 7, 581–587
Spencer, T.M., Gordon-Kamm, W.J., Daines, R.J., Start, W.G., Lemaux, P.G. (1990) Bialaphos selection of stable transformants from maize cell culture. Theor. Appl. Genet. 79, 625–631
Tachibana, K., Kaneko, K. (1986) Development of a new herbicide, Bialaphos. J. Pestic. Sci. 11, 297–304
Tachibana, K., Watanabe, T., Sekizawa, Y., Takematsu, T. (1986) Accumulation of ammonia in plants treated with Bialaphos. J. Pestic. Sci. 11, 33–37
Thompson, C.J., Movya, N.R., Tizard, R., Crameri, R., Davies, J.E., Lauwereys, M., Botterman, J. (1987) Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopus. EMBO J. 6, 2519–2524
Ziegler, C., Wild, A. (1989) The effect of Bialaphos on ammonium-assimilation and photosynthesis. Z. Naturforsch, 44c, 103–108
Author information
Authors and Affiliations
Additional information
The authors thank G. Pace for critical reading of the manuscript and Jeanette Tyler for technical assistance.
Rights and permissions
About this article
Cite this article
Kramer, C., DiMaio, J., Carswell, G.K. et al. Selection of transformed protoplast-derived Zea mays colonies with phosphinothricin and a novel assay using the pH indicator chlorophenol red. Planta 190, 454–458 (1993). https://doi.org/10.1007/BF00224783
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00224783