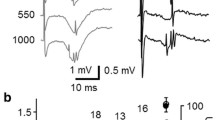
Abstract
The present study determined the membrane and synaptic properties of neurons in the rat subiculum. Using the in vitro hippocampal slice preparation, intracellular recordings were obtained from 91 subicular neurons. Membrane properties and morphological characteristics were similar to those reported for hippocampal pyramidal neurons. Two categories of subicular neurons were distinguished based on their response to a depolarizing current pulse. One type of neuron showed bursting behavior and the second type was characterized as regular firing. Analysis of the charging functions during hyperpolarizing current pulses yielded a mean τ0 and τ1 for subicular neurons of about 13 ms and 0.60 ms, respectively. Using the model of an equivalent cylinder, the mean dendrite-to-soma conductance ratio (ρ) was estimated at 6.0 and electrotonic length constant (L) at 0.7. There was no difference in these values between bursting and regular firing neurons. Tetrodotoxin-resistant potentials (presumed calcium hump/spike) were evoked from bursting subicular neurons at lower current intensities than CA1 pyramidal neurons. Calcium humps could only be evoked from about half the regular firing subicular neurons. Subicular cells showed an excitatory/inhibitory postsynaptic potential (EPSP/IPSP) sequence in response to electrical stimulation in different layers of the CA1 area. An EPSP could also be evoked from stimulation of the superficial or deep layers of the presubiculum and was attributed to activation of entorhinal fibers of passage. At high stimulation intensity, an antidromic spike was often evoked following stimulation in the presubicular area or CA1 alveus. The evoked EPSPs were blocked by addition of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to the bathing medium. In magnesium-free, CN-QX bathing solution, a longer lasting depolarization was recorded; this response was blocked by application of a N-methyl-d-aspartate (NMDA) receptor antagonist (AP5). Iontophoretic application of glutamate or quisqualate (10 mM) along the soma-dendritic axis of subicular neurons leads to either a short-latency depolarization or a burst of action potentials. Application of 10 mM GABA near the recording site usually produced a hyperpolarization, which, at times, was mixed with a depolarization. Mixed hyperpolarizing/depolarizing responses were observed when GABA was applied to the basal or apical dendritic areas. There were no significant differences in the synaptic properties or responses to drug application between bursting and regular firing neurons. These results indicate that subicular neurons (1) are composed of a heterogeneous population of cell types, (2) have similar electrical properties to other hippocampal principal neurons, (3) receive glutaminergic synapses from CA1 and entorhinal cortical neurons, (4) project to the presubicular area and fornix (via the alveus), (5) are inhibited by local circuit neurons, and (6) display complex responses to GABA.
Similar content being viewed by others
References
Allger BE, Nicoll RA (1979) GABA-mediated biphasic inhibitory responses in hippocampus. Nature 281:315–317
Alger BE, Nicoll RA (1982) Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol (Lond) 328:105–123
Amaral DG (1987) Memory: anatomical organization of candidate brain regions. In: Mountcastle V, Plum F, Geiger S (eds) Higher functions of the brain, part 1. (Handbook of physiology, sect 1, The nervous system, vol. V). American Physiological Society, Bethesda, Md, pp 211–294
Andersen P, Dingledine R, Gjerstad L, Langmoen IA, Mosfeldt Laursen A (1980) Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol (Lond) 305:279–296
Barnes CA, McNaughton BL (1980) Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol (Lond) 309:473–485
Barnes CA, McNaughton BL, Mizumori SJY, Leonard BW, Lin L-H (1990) Comparison of spatial temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res 83:287–300
Bartesaghi R, Gessi T (1986) Hippocampal output to the subicular cortex: an electrophysiological study. Exp Neurol 92:114–133
Berger TW, Swanson GW, Milner TA, Lynch GS, Thompson RF (1980) Reciprocal anatomical connections between hippocampus and subiculum in the rabbit: Evidence for subicular innervation of regio superior. Brain Res 183:265–276
Blackstad TW (1956) Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol 105:417–537
Brown TH, Fricke RA, Perkel DH (1981a) Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol 46:812–827
Brown TH, Perkel DH, Norris JC, Peacock JH (1981b) Electrotonic structure and specific membrane properties of mouse dorsal root ganglion neurons. J Neurophysiol 45:1–15
Chagnac-Amitai Y, Connors BW (1989) Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol 62:1149–1162
Collingridge GL, Kehl SJ, McLennan H (1983) Excitatory amino acids in synaptic transmission in the schaffer collateral-commisural pathway of the rat hippocampus. J Physiol (Lond) 334:33–46
Davies DC, Wilmott AC, Mann DMA (1988) Senile plaques are concentrated in the subicular region of the hippocampal formation in Alzheimer's disease. Neurosci Lett 94:228–233
Djorup A, Jahnsen H, Mosfeldt Laursen A (1981) The dendritic response to GABA in CA1 of the hippocampal slice. Brain Res 219:196–201
Finch DM, Babb TL (1980) Neurophysiology of the caudally directed hippocampal efferent system in the rat: Projections to the subicular complex. Brain Res 197:11–26
Finch DM, Wong EE, Derian EL, Babb TL (1986) Neurophysiology of limbic system pathways in the rat: projections from the subicular complex and hippocampus to the entorhinal cortex. Brain Res 397:205–213
Fricke RA, Prince DA (1984) Electrophysiology of dentate gyrus granule cells. J Neurophysiol 51:195–209
Hotson JR, Prince DA (1980) A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol 43:409–419
Johnston D (1981) Passive cable properties of hippocampal CA3 pyramidal neurons. Cell Mol Neurobiol 1:41–55
Jones RSG, Heinemann U (1988) Synaptic and intrinsic responses of medial entorhinal cortical cells in normal and magnesium-free medium in vitro. J Neurophysiol 59:1476–1496
Knowles WD, Schwartzkroin PA (1981) Axonal ramifications of hippocampal CA1 pyramidal cells. J Neurosci 1:1236–1241
Kohler C (1985) Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol 236:504–522
Lacaille J-C, Kunkel DD, Schwartzkroin PA (1989) Electrophysiological and morphological characterization of hippocampal interneurons. In: Chan-Palay V, Kohler C (eds) The hippocampus: new vistas. Liss,New York, pp 287–305
Lacaille J-C, Williams S (1990) Membrane properties of interneurons in stratum oriens-alveus of the CA1 region of rat hippocampus in vitro. Neuroscience 36:349–359
Lopes da Silva FH, Witter MP, Boeijinga PH, Lohman AHM (1990) Anatomic organization and physiology of the limbic cortex. Physiol Rev 70:453–511
Lorente de No R (1934) Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol 46:113–177
McCormick DA (1992) Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 39:337–388
McCormick DA, Connors BW, Lightfall JW, Prince DA (1985) Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54:782–806
Miller RJ (1987) Multiple calcium channels and neuronal function. Science 235:46–52
Monaghan DT, Cotman CW (1985) Distribution of N-methyl-d-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci 5:2909–2919
Mueller AL, Taube JS, Schwartzkroin PA (1984) Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to GABA in rabbit hippocampus studied in vitro. J Neurosci 4:860–867
Nadler JV, Vaca KW, White WF, Lynch GS, Cotman CW (1976) Aspartate and glutamate as possible transmitters of excitatory hippocampal afferents. Nature 260:538–540
Newberry NR, Nicoll RA (1984) A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cell in vitro. J Physiol (Lond) 348:239–254
Newberry NR, Nicoll RA (1985) Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol (Lond) 360:161–185
Nicoll RA, Malenka RC, Kauer JA (1990) Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev 70:513–565
Rall W (1969) Time constants and electrotonic length of membrane cylinder and neurons. Biophys J 9:1483–1508
Robitaille R, Charlton MP (1992) Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci 12:297–305
Scharfman HE, Schwartzkroin PA (1988) Electrophysiology of morphologically identified mossy cells of the dentate hilus recorded in guinea pig hippocampal slices. J Neurosci 8:3812–3821
Schwartzkroin PA (1975) Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res 85:423–436
Schwartzkroin PA (1977) Further characteristics of hippocampal CA1 cells in vitro. Brain Res 128:53–68
Schwartzkroin PA (1983) Local circuit considerations and intrinsic neuronal properties involved in hyperexcitability and cell synchronization. In: Van Gelder NM, Jasper HH (eds) Basic mechanisms of neuronal hyperexcitability. Liss,New York, pp 75–108
Schwartzkroin PA, Slawsky M (1977) Probable calcium spikes in hippocampal neurons. Brain Res 135:157–161
Schwartzkroin PA, Stafstrom CE (1980) Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science 210:1125–1126
Sharp P, Ranck JB Jr, Muller RU (1990) Direction and location correlates of cell firing in the rat subiculum. Soc Neurosci Abstr 16:737
Sorensen KE, Shipley MT (1979) Projections from the subiculum to the deep layers of the ipsilateral presubicular and entorhinal cortices in the guinea pig. J Comp Neurol 188:313–334
Spruston N, Johnston D (1992) Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J Neurophysiol 67:508–529
Staley KJ and Mody I (1992) Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol 68:197–212
Swanson LW, Wyss JM, Cowan WM (1978) An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol 181:681–714.
Swanson LW, Cowan WM (1977) An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172:49–84
Taube JS, Cotman CW (1991) Intracellular recordings of rat subicular neurons. Soc Neurosci Abstr 17:1037
Taube JS, Schwartzkroin PA (1986) Ineffectiveness of organic calcium channel blockers in antagonizing long-term potentiation. Brain Res 379:275–285
Taube JS, Schwartzkroin PA (1987) Hyperpolarizing responses to application of glutamate in hippocampal CA1 pyramidal neurons. Neurosci Lett 78:85–90
Taube JS, Schwartzkroin PA (1988) Mechanisms of long-term potentiation: EPSP/spike dissociation, intradendritic recordings, and glutamate sensitivity. J Neurosci 8:1632–1644
Taube JS, Muller RU, Ranck JB Jr (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10:420–435
Thalmann RH, Peck EJ, Ayala GF (1981) Biphasic response of hippocampal pyramidal neurons to GABA. Neurosci Lett 21; 319–324
Turner DA (1984) Segmental cable evaluation of somatic transients in hippocampal neurons (CA1, CA3, and dentate). J Biophys 46:73–84
Uemura E (1985) Age-related changes in the subiculum of Macaca mulatta: dendritic branching pattern. Exp Neurol 87:412–427
Van Groen T, Lopes da Silva FH (1986) Organization of the reciprocal connections between the subiculum and the entorhinal cortex in the cat II. An electrophysiological study. J Comp Neurol 251:111–120
Van Groen T, Van Haren FJ, Witter MP, Groenewegen HJ (1986) The organization of the reciprocal connections between the subiculum and the entorhinal cortex in the cat I. A neuroanatornical tracing study. J Comp Neurol 250:485–497
Walther H, Lambert JDC, Jones RSG, Heinemann U, Hamon B (1986) Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett 69:156–161
Witter MP, Groenewegen HJ (1990) The subiculum: cytoarchitectonically a simple structure, but hodologically complex. Prog Brain Res 83:47–58
Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM (1989) Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol 33:161–253
Wong RKS, Prince DA (1978) Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res 159:385–390
Wong RKS, Prince DA (1981) Afterpotential generation in hippocampal pyramidal cells. J Neurophysiol 45:86–97
Wong RKS, Watkins DJ (1982) Cellular factors influencing GABA response in hippocampal pyramidal cells. J Neurophysiol 48:938–951
Wong RKS, Traub RD, Miles R (1984) Epileptogenic mechanisms as revealed by studies of the hippocampal slice. In: Schwartzkroin PA, Wheal H (eds) Electrophysiology of Epilepsy. Acaademic London pp 253–275
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taube, J.S. Electrophysiological properties of neurons in the rat subiculum in vitro. Exp Brain Res 96, 304–318 (1993). https://doi.org/10.1007/BF00227110
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227110