Summary
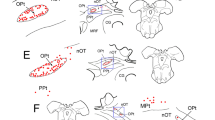
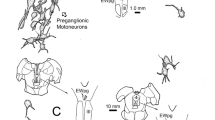
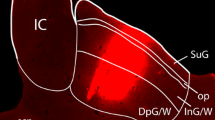
The horseradish peroxidase (HRP) retrograde transport method was used to identify brainstem afferents to the cerebellar flocculus in the pigmented rat. Injections of the enzyme were made through recording microelectrodes, making it possible to localize the injection site by physiological criteria. Clearly, the largest number of afferents arise from the bilateral vestibular and perihypoglossal nuclei and from the contralateral dorsal cap (of Kooy) of the inferior olive. Additionally, a substantial number arise bilaterally from: (1) the nucleus reticularis tegmenti pontis (NRTP); (2) several of the cranial motor nuclei including the abducens, retrofacial and facial nuclei and the nucleus ambiguus; (3) the rostral part of the lateral reticular nucleus (subtrigeminal nucleus); (4) the raphe pontis and raphe magnus and (5) neurons intercalated among the medial longitudinal fasciculus (MLF) just rostral to the hypoglossal nucleus and another group rostral to the abducens nucleus.
The basilar pontine nuclei contained a large number of lightly labeled neurons in all flocculus injections which were discretely located within the dorsolateral, lateral and medial divisions. These areas were labeled bilaterally but with a slight contralateral preponderance. Injection into the flocculus, but involving the adjacent ventral paraflocculus, produced a heavier labeling of pontine neurons with a slightly different distribution. Therefore, we tentatively conclude that the flocculus receives input from these pontine visual centers (dorsolateral, lateral and medial nuclei), perhaps through collateral projections from neurons projecting to the paraflocculus.
The present study demonstrates strong similarities between the rat and other species studied (e.g., rabbit, cat, monkey) in terms of the brainstem nuclei projecting to the flocculus. Most noticeable in quantitative terms are the pathways known to mediate vestibular (vestibular and perihypoglossal nuclei) and visual (optokinetic) information (e.g., NRTP). Additionally, we can provide morphological evidence that the midline and paramedian pontine tegmentum, identified in the cat and monkey as containing saccade-related neurons, send large numbers of projections to the rat flocculus. Given these similarities, the rat may be a suitable animal model in which to study the pathways underlying visual-vestibular interaction and saccadic mechanisms in the flocculus.
Similar content being viewed by others
References
Alley K, Baker R, Simpson JI (1975) Afferents to the vestibulocerebellum and the origin of the visual climbing fibers in the rabbit. Brain Res 98: 582–589
Angaut P, Brodal A (1967) The projection of the “vestibulocerebellum” onto the vestibular nuclei in the cat. Arch Ital Biol 105: 441–479
Baker J, Gibson A, Glickstein M, Stein J (1976) Visual cells in the pontine nuclei of the cat. J Physiol (Lond) 255: 415–433
Blanks RHI, Palay S (1978) The location and form of efferent vestibular neurons in the rat. Anat Rec 190: 341A
Blanks RHI, Precht W (1983) Optokinetic and vestibular responses of neurons in the cerebellar flocculus of the alert, pigmented rat. Exp Brain Res (in press)
Blanks RHI, Torigoe Y (1983) A pontine cell group associated with the medial longitudinal fasciculus which conveys vestibular impulses to the cerebellar flocculus in cat and rat. Soc Neurosci (Abstr) (in press)
Brodal A (1940) Experimentelle Untersuchungen über die olivocerebellare Lokalisation. Z Ges Neurol Psychiat 169: 1–153
Brodal A (1943) The cerebellar connections of the nucleus reticularis lateralis (nucleus funiculi lateralis) in the rabbit and cat. Experimental investigations. Acta Psychiat Neurol Scand 18: 171–233
Brodal A (1952) Experimental demonstration of cerebellar con-nexions from the perihypoglossal nuclei (nucleus intercalatus, nucleus praepositus hypoglossi and nucleus of Roller) in the cat. J Anat (Lond) 86: 110–120
Brodal A, Høivik B (1964) Site and mode of termination of primary vestibulocerebellar fibers in the cat. An experimental study with silver impregnation methods. Arch Ital Biol 102: 1–21
Brodal A, Kawamura K (1980) Olivocerebellar projection: A review. Adv Anat Embryol Cell Biol 64: 1–140
Brodal A, Pompeiano O (1957) The vestibular nuclei in the cat. J Anat (Lond) 91: 438–454
Brodal A, Torvik A (1957) Über den Ursprung der sekundären vestibulocerebellären Fasern bei der Katze. Eine experimentell anatomische Studie. Arch Psychiat Neurol 195: 550–567
Brodal P (1982) Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the Rhesus monkey. J Comp Neurol 204: 44–55
Burne RA, Eriksson MA, Saint-Cyr JA, Woodward DJ (1978a) The organization of the pontine projection to lateral cerebellar areas in the rat: Dual zones in the pons. Brain Res 139: 340–347
Burne RA, Mihailoff GA, Woodward DJ (1978b) Visual corticopontine input to the paraflocculus: A combined autoradiographi and horseradish peroxidase study. Brain Res 143: 139–146
Burne RA, Woodward DJ (1977) The ventral paraflocculus: a site of visual cortical and tectal input. Soc Neurosci (Abstr) 3: 55
Carpenter MB, Stein BM, Peter P (1972) Primary vestibulocerebellar fibers in the monkey: Distribution of fibers arising from distinctive cell groups of the vestibular ganglia. Am J Anat 135: 221–250
Cohen B, Henn V (1972) The origin of quick phases of nystagmus in the horizontal plane. Bibl Ophthalmol 82: 36–55
Corbin KB, Hinsey JC (1935) Intramedullary course of the dorsal root fibers of each of the first four cervical nerves. J Comp Neurol 63: 119–126
Curthoys IS, Nakao S, Markham CH (1981) Cat medial pontine reticular neurons related to vestibular nystagmus: Firing pattern, location and projection. Brain Res 222: 75–94
de Olmos J, Hardy H, Heimer L (1978) The afferent connections of the main and the accessory olfactory bulb information in the rat: an experimental HRP-study. J Comp Neurol 181: 213–244
Graybiel AM (1974) Visuo-cerebellar and cerebello-visual connections involving the ventral lateral geniculate nucleus. Exp Brain Res 20: 303–306
Graybiel AM (1977) Organization of oculomotor pathways in the cat and rhesus monkey. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neurons. Developments in neuroscience, vol 1. Elsevier/North-Holland Biomedical Press, New York, pp 79–88
Hess R, Simpson JI (1978) Visual and somatosensory messages to the rabbit's cerebellar flocculus. Neurosci Lett [Suppl] 1: 146
Hoddevik GH (1977) The pontine projection to the flocculonodular lobe and the paraflocculus studied by means of retrograde transport of horseradish peroxidase in the rabbit. Exp Brain Res 30: 511–526
Hoddevik GH (1978) The projection from nucleus reticularis tegmenti pontis onto the cerebellum in the cat. Anat Embryol 153: 227–242
Hoddevik GH, Brodal A (1977) The olivocerebellar projection studied with the method of retrograde axonal transport of horseradish peroxidase. V. The projections to the flocculonodular lobe and the paraflocculus in the rabbit. J Comp Neurol 176: 269–280
Holstege G, Collewijn H (1982) The efferent connections of the nucleus of the optic tract and the superior colliculus in the rabbit. J Comp Neurol 209: 139–175
Ito M (1982) Cerebellar control of the vestibulo-ocular reflex — around the flocculus hypothesis. Ann Rev Neurosci 5: 275–296
Jansen J, Brodal A (1954) Aspects of cerebellar anatomy. Tanum, Oslo
Kawasaki T, Kato I, Sato Y, Mizukoshi K (1982) The brain-stem projection to the cerebellar flocculus relevant to optokinetic responses in cat. Ann NY Acad Sci 374: 455–464
Keller EL, Precht W (1978) Persistence of visual response in vestibular nucleus neurons in cerebellectomized cat. Exp Brain Res 32: 591–594
Kotchabhakdi N, Walberg F (1977) Cerebellar afferents from neurons in motor nuclei of cranial nerves demonstrated by retrograde axonal transport of horseradish peroxidase. Brain Res 137: 158–163
Kotchabhakdi N, Walberg F (1978) Cerebellar afferent projections from the vestibular nuclei in the cat: an experimental study with the method of retrograde axonal transport of horseradish peroxidase. Exp Brain Res 31: 591–604
Larsell O (1970) The comparative anatomy and histology of the cerebellum from monotremes through apes. University of Minnesota Press, Minneapolis, pp 31–58
Lisberger SG, Fuchs AF (1978) Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movement and passive head rotation. J Neurophysiol 41: 733–763
Maekawa K, Kimura M (1980) Mossy fiber projections to the cerebellar flocculus from the extraocular muscle afferents. Brain Res 191: 313–325
Maekawa K, Kimura M (1981) Electrophysiological study of the nucleus of the optic tract that transfers optic signals to the nucleus reticularis tegmenti pontis — the visual mossy fiber pathway to the cerebellar flocculus. Brain Res 211: 456–462
Maekawa K, Simpson JI (1973) Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J Neurophysiol 36: 649–666
Maekawa K, Takeda T (1978) Origin of the mossy fiber projection to the cerebellar flocculus from the optic nerves in rabbit. In: Ito M, Tsukahara N, Kubota K, Yagi K (eds) Integrative control functions of the brain, vol 1. Kodansha Ltd., Tokyo; Elsevier/North-Holland Biomedical Press, Amsterdam, pp 93–95
Maekawa K, Takeda T (1979) Origin of the non-visual mossy fiber afferents to the cerebellar flocculus in the pons of rabbits: A HRP study. In: Ito M, Tsukahara N, Kubota K, Yagi K (eds) Integrative control functions of the brain, vol 2. Kodansha Ltd., Tokyo; Elsevier/North-Holland Biomedical Press, Amsterdam, pp 113–114
Maekawa K, Takeda T, Kimura M (1981) Neural activity of nucleus reticularis tegmenti pontis — The origin of visual mossy fiber afferents to the cerebellar flocculus of rabbits. Brain Res 210: 17–30
McCrea RA, Baker R, Delgado-Garcia J (1979) Afferent and efferent organization of the prepositus hypoglossi nucleus. In: Granit R, Pompeiano O (eds) Reflex control of posture and movement. Progress in brain research, vol 50. Elsevier/North-Holland Biomedical Press, Amsterdam, pp 653–665
Mergner T, Anastasopoulos D, Becker W (1982) Neuronal responses to horizontal neck deflection in the group region of the cat's medullary brainstem. Exp Brain Res 45: 196–206
Mihailoff GA, McArdle CB, Adams CE (1981) The cytoarchitecture, cytology and synaptic organization of the basilar pontine nuclei in the rat. I. Nissl and Golgi studies. J Comp Neurol 195: 181–201
Miles FA, Lisberger SG (1981) Plasticity in the vestibulo-ocular reflex: a new hypothesis. Ann Rev Neurosci 4: 273–299
Miyashita Y, Ito M, Jastreboff PJ, Maekawa K, Nagao S (1980) Effect upon eye movements of rabbits induced by severance of mossy fiber visual pathway to the cerebellar flocculus. Brain Res 198: 210–215
Nakao S, Curthoys IS, Markham CH (1980) Eye movement related neurons in the cat pontine reticular formation: projection to the flocculus. Brain Res 180: 291–299
Precht W (1978) Neuronal operations in the vestibular system. Springer, Berlin Heidelberg New York, pp 1–226
Precht W, Cazin L, Blanks RHI, Lannou J (1982) Anatomy and physiology of the optokinetic pathways to the vestibular nuclei in the rat. In: Roucoux A, Crommelinck M (eds) Physiological and pathological aspects of eye movements. Junk, The Hague Boston London, pp 418–423
Taber E, Brodal A, Walberg F (1960) The raphe nuclei of the brainstem in the cat. I. Normal topography and cytoarchitecture and general discussion. J Comp Neurol 114: 161–188
Taber-Pierce E, Hoddevik GH, Walberg F (1977) The cerebellar projection from the raphe nuclei in the cat as studied with the method of retrograde transport of horseradish peroxidase. Anat Embryol 152: 73–87
Voogd J, Bigaré F (1980) Topographical distribution of olivary and cortico-nuclear fibers in the cerebellum: A review. In: Courville J, de Montigny C, Lamarre Y (eds) The inferior olivary nucleus: Anatomy and physiology. Raven Press, New York, pp 207–234
Waespe W, Henn V (1981) Visual-vestibular interaction in the alert monkey. II. Purkinje cell activity. Exp Brain Res 43: 349–360
Walberg F, Kotchabhakdi N, Hoddevik GH (1979) The olivocerebellar projections to the flocculus and paraflocculus in the cat, compared to those in the rabbit. A study using horseradish peroxidase as a tracer. Brain Res 161: 389–398
Wilson VJ, Maeda M, Franck JI (1975) Input from neck afferents to the cat flocculus. Brain Res 89: 133–138
Wilson VJ, Maeda M, Franck JI, Shimazu H (1976) Mossy fiber neck and second order labyrinthine projections to cat flocculus. J Neurophysiol 39: 301–310
Winfield JA, Hendrickson A, Kimm J (1978) Anatomical evidence that the medial terminal nucleus of the accessory optic tract in mammals provides a visual mossy fiber input to the flocculus. Brain Res 151: 175–182
Yamamoto M (1979) Topographical representation in rabbit cerebellar flocculus for various afferent inputs from the brainstem investigated by means of retrograde axonal transport of horseradish peroxidase. Neurosci Lett 12: 29–34
Author information
Authors and Affiliations
Additional information
Supported by USPHA Research Grant EY03018 and Research Career Development Award to R.H.I.B. (EY00160) from the National Eye Institute and grants 3.505.79 and 3.616.80 to W.P. from the Swiss National Science Foundation. Travel funds for this work were provided by NATO Grant (1918)/1047 (79) AG
Rights and permissions
About this article
Cite this article
Blanks, R.H.I., Precht, W. & Torigoe, Y. Afferent projections to the cerebellar flocculus in the pigmented rat demonstrated by retrograde transport of horseradish peroxidase. Exp Brain Res 52, 293–306 (1983). https://doi.org/10.1007/BF00236639
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00236639