Summary
In birds, the accessory optic system (AOS) includes two nuclei: the nucleus ectomamillaris (nEM) and the pretectal nucleus superficialis synencephali (nSS). The role of the nSS in the production of a horizontal optokinetic nystagmus (OKN) was studied in the pigeon, by comparing the OKN before and after a unilateral lesion of this nucleus. The lesions were performed either by electrolysis or by local application of kainic acid (KA); the KA lesions gave more stable modifications of the OKN than the electrolytic lesions. A quantitative analysis of the slow-phase velocity (¯V) of the OKN was carried out on the animals receiving KA lesions.
-
(1)
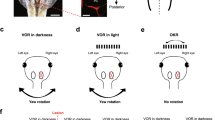
Lesion of the nSS provokes the almost total disappearance of the OKN for stimulation of the contralateral eye in the temporo-nasal direction, and a reduction of the OKN for stimulation in the nasotemporal direction. Thus, the nSS is essential for the production of the OKN in the temporo-nasal direction, but it also participates in the production of the OKN in the naso-temporal direction (slow-phase direction).
-
(2)
The same lesion produces a large increase of the OKN (¯V) when the ipsilateral eye is stimulated in the temporo-nasal direction, and a smaller increase following stimulation in the naso-temporal direction. These increases suggest some kind of inhibitory (or disfacilitatory) interactions between the nSS (or the associated system) on one side, and the contralateral optokinetic centers.
-
(3)
The lesion of one nSS does not provoke a deficit when the stimulation is binocular. This result probably reflects the combined effect of both monocular inputs.
-
(4)
After a pretectal KA injection, a spontaneous nystagmus of the contralateral eye, in the nasotemporal direction, can be seen for several hours. The mechanism is still unknown, but it might be rela2ted to a reverse optokinetic after nystagmus (ROKAN).
The anatomical and physiological data so far available consistently support the hypothesis of a functional equivalence between the nSS in birds and the nucleus of the optic tract in mammals.
Similar content being viewed by others
References
Ballas I, Hoffmann KP, Wagner HJ (1981) Retinal projection to the nucleus of the optic tract in the cat as revealed by retrograde transport of horseradish peroxidase. Neurosci Lett 26: 197–202
Berman NI (1977) Connections of the pretectum in the cat. J Comp Neurol 174: 227–254
Bondy SC, Purdy JL (1977) Putative neurotransmitters of the avian visual pathway. Brain Res 119: 417–426
Brauth SE, Karten HJ (1977) Direct accessory optic projections to the vestibulo-cerebellum: A possible channel for oculomotor control systems. Exp Brain Res 28: 73–84
Brecha N, Karten HJ (1978) Projection of the accessory optic nuclei and vestibular nuclei upon the oculomotor nuclear complex in pigeon. Ann Rec 190: 605–606
Brecha N, Karten HJ, Hunt SP (1980) Projections of the nucleus of the basal optic root in the pigeon: An autoradiographic and horseradish peroxidase study. J Comp Neurol 189: 615–670
Cazin L, Precht W, Lannou J (1980a) Optokinetic responses of pretectal neurons (PT) and visual-vestibular convergence in n. Reticularis Tegmenti Pontis (NRTP) of the rat. Neurosci Lett [Suppl] 5: 182
Cazin L, Precht W, Lannou J (1980b) Pathways mediating optokinetic responses of vestibular nucleus neurons in the rat. Pflügers Arch 384: 19–29
Clarke PGH (1977) Some visual and other connections to the cerebellum of the pigeon. J Comp Neurol 174: 535–552
Clarke RJ, Ikeda H (1978) Properties of neurons in the nucleus of the optic tract of the pretectum in the cat. J Physiol (Lond) 275: 46P-47P
Collewijn H (1975a) Oculomotor areas in the rabbit's midbrain and pretectum. J Neurobiol 6: 3–22
Collewijn H (1975b) Direction-selective units in the rabbit's nucleus of the optic tract. Brain Res 100: 489–508
Flohr H, Precht W (1981) Lesion-induced neuronal plasticity in sensorimotor systems. Springer, Berlin Heidelberg New York
Gioanni H, Rey J, Villalobos J, Bouyer JJ, Gioanni Y (1981) Optokinetic nystagmus in the pigeon (columba livia): I. Study in monocular and binocular vision. Exp Brain Res 44: 362–370
Giolli RA (1961) An experimental study of the accessory optic tracts (transpeduncular tracts and anterior accessory optic tracts) in the rabbit. J Comp Neurol 117: 77–85
Giolli RA (1963) An experimental study of the accessory optic system in the cynomolgus monkey. J Comp Neurol 121: 89–108
Giolli RA, Guthrie MD (1969) The primary optic projections in the rabbit. An experimental degeneration study. J Comp Neurol 136: 99–126
Graybiel AM (1974) Some efferents of the pretectal region in the cat. Anat Rec 178: 365
Hayhow WR (1959) An experimental study of the accessory optic fiber system in the cat. J Comp Neurol 113: 281–313
Hayhow WR, Webb C, Jerve A (1960) The accessory optic fiber system in the rat. J Comp Neurol 115: 187–215
Henn V, Cohen B, Young L (1980) Visual vestibular interaction in motion perception and the generation of nystagmus. Neurosci Res Program Bull 18
Hoffmann KP, Behrend K, Schoppmann (1976) A direct afferent visual pathway from the nucleus of the optic tract to the inferior olive in the cat. Brain Res 115: 150–153
Hoffmann KP, Schoppmann A (1975) Retinal input to direction selective cells in the nucleus tractus opticus of the cat. Brain Res 99: 359–366
Hoffmann KP, Schoppmann A (1981) A quantitative analysis of the direction specific response of neurons in the cat's nucleus of the optic tract. Exp Brain Res 42: 146–157
Holstege G, Collewijn H (1980) The efferent connections of the nucleus of the optic tract of the rabbit. Neurosci Lett [Suppl] 5: 183
Karten HJ, Hodos W (1967) A stereotaxic atlas of the brain of the pigeon (columba livid). Johns Hopkins Press, Baltimore
Kostovic I (1971) The terminal distribution of accessory optic fibers in the rat. Brain Res 31: 202–206
Kuhlenbeck H (1939) The development and structure of the pretectal cell masses in the chick. J Comp Neurol 71: 361–387
Kuhlenbeck H, Miller RN (1942) The pretectal region of the rabbit's brain. J Comp Neurol 76: 323–365
Lin H, Giolli RA (1979) Accessory optic system of rhesus monkey. Exp Neurol 63: 163–176
McGeer G, Olney JW, Mc Geer PL (1978) Kainic acid as a tool in neurobiology. Raven Press, New York
McKenna OC, Wallman J (1981) Identification of avian brain regions responsive to retinal slip using 2-deoxyglucose. Brain Res 210: 455–460
Maekawa K, Kimura M (1981) Electrophysiological study of the nucleus of the optic tract that transfers optic signals to the nucleus reticularis tegmenti pontis. The visual mossy fiber pathway to the cerebellar flocculus. Brain Res 211: 456–462
Maekawa K, Takeda T (1977) Afferent pathways from the visual system to the cerebellar flocculus of the rabbit. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neurons, vol I. Elsevier/North-Holland Biomedical Press, Amsterdam
Marg E (1964) Neurophysiology of the accessory optic system. Ann NY Acad Sci 117: 35–52
Martinoya C, Rivaud S, Boch S (1981) Eye movements in pigeons: Participation in binocular fixation and visual pursuit. J Physiol (Lond) 320: 20–21P
Mizuno N, Mochizuki K, Akimoto C, Matsushima R (1973) Pretectal projections to the inferior olive in the rabbit. Exp Neurol 39: 498–506
Neverov VP, Bures J (1979) Asymmetry of optokinetic trace phenomena induced by unilateral 6-OHDA lesion of substantia nigra in the rabbit. Neurosci Lett 13: 301–305
Neverov VP, Ueda M, Bures J (1976) The effect of cortical and collicular spreading depression on the optokinetic and reversive postoptokinetic nystagmus in rabbits. Brain Res 115: 318–323
Neverov VP, Sterc J, Bures J (1980) Electrophysiological correlates of the reversed postoptokinetic nystagmus in the rabbit: Activity of vestibular and floccular neurons. Brain Res 189: 355–367
Pasik P, Pasik T, Bender MB (1969a) The pretectal syndrome in monkeys. I. Disturbances of gaze and body posture. Brain 92: 521–534
Pasik T, Pasik P, Bender MB (1969b) The pretectal syndrome in monkeys. II. Spontaneous and induced nystagmus, and “lightning” eye movements. Brain 92: 871–884
Precht W, Strata P (1980) On the pathway mediating optokinetic responses in vestibular nuclear neurons. Neuroscience 5: 777–787
Rendahl H (1924) Embryologische and morphologische Studien über das Zwischenhirn beim Huhn. Acta Zool Pathol Antverp 5: 241–344
Repérant J (1973) Nouvelles données sur les projections visuelles chez le pigeon (columba livia). J Hirnforsch 14: 151–188
Repérant J (1978) Organisation anatomique du système visuel des vertébrés approche evolutive. Thesis, Paris
Scalia R (1972) The termination of retinal axons in the pretectal regions of mammals. J Comp Neurol 145: 223–258
Simpson JI, Soodak RE, Hess R (1979) The accessory optic system and its relation to the vestibulo-cerebellum. Prog Brain Res 50: 715–724
Terasawa K, Otani K, Yamada J (1979) Descending pathways of the nucleus of the optic tract in the rat. Brain Res 173: 405–417
Walley RE (1967) Receptive fields in the accessory optic system of the rabbit. Exp Neurol 17: 27–43
Watkins JC (1978) Excitatory amino acids. In: Mc Geer EG, Olney JW, Mc Gear PL (eds) Kainic acid as a tool in neurobiology. Raven Press, New York, pp 37–69
Weber JT, Harting JK (1980) The efferent projections of the pretectal complex: Autoradiographic and horseradish peroxidase analysis. Brain Res 194: 1–28
Winfield JA, Hendrickson A, Kimm J (1978) Anatomical evidence that the medial nucleus of the accessory optic tract in mammals provides a visual mossy fiber input to the flocculus. Brain Res 151: 175–182
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gioanni, H., Rey, J., Villalobos, J. et al. Optokinetic nystagmus in the pigeon (Columba livia) II. Role of the pretectal nucleus of the accessory optic system (AOS). Exp Brain Res 50, 237–247 (1983). https://doi.org/10.1007/BF00239188
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00239188