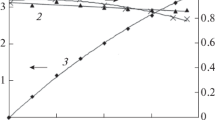
Abstract
The long history of the synthesis of hydrogen peroxide via the cathodic reduction of oxygen in caustic soda catholyte is reviewed. Recent progress is analysed on the electrochemical syntheses of mixtures of caustic soda and hydrogen peroxide in various by-weight ratios from 2.3: 1 NaOH to H2O2 to about 1 : 1. The analysis presented focuses primarily on published work concerning planar fuel cell type electrodes in membrane-divided cells and particulate bed electrodes in cells employing microporous separators with well-defined anolyte-to-catholyte flows. Potential ancillary technology for changing the ratios of products is also discussed. One configuration of the processes described encompasses the simultaneous near 50/50 use of two variations of generation technology. A highly desired product, for instance 1.2: 1 NaOH to H2O2, may be formed using the catholyte product of a membrane or diaphragm cell with a caustic anolyte as the catholyte feed stream for a membrane cell with an acidic anolyte. Although the particulate bed cathode approach has reached commercial trials, the planar cathode membrane cell approach may prove a difficult process to develop as the performance of electrodes optimized for realistic hydraulic depths may prove very different to that of electrodes used in small scale laboratory development.
Similar content being viewed by others
References
R. P. Singh (ed.), ‘The Bleaching of pulp’, Tappi Press, Atlanta, GA (1979).
PERP Report, ‘Hydrogen Peroxide: Commercial Status’, Chem Systems, Inc., Tarrytown, New York (June 1991).
Y. R. Chin, SRI International, Inc., Chemical Industries Center, ‘Hydrogen Peroxide by the cathodic reduction of oxygen’, Menlo Park, CA (March 1992).
‘Cost of H2O2’, Chemical Marketing Reporter (Dec. 1993).
Private communication: excerpts from ‘Analysis of Economics of Hydrogen Peroxide Production’, CPI Consulting, Inc., White Plains, NY (1993).
P. B. Walsh, Tappi J. 74(1) (1991) 81.
A. Barnes and R. Coghill, Appita J. 43(2) (1990) 143.
V. R. Parthasarathey, R. Klein, V. Sundaran and H. Jameel, Tappi J. 73(1) (1990) 177.
R. C. Lachapelle, W. G. Strunk and R. J. Klein, ibid.Tappi J. 75(7) (1992) 181.
O. Helming, H. U. Suess, J. Meier and M. Berger, ibid.Tappi J. 72(7) (1989) 55.
P. Stockburger, ibid.Tappi J. 76(3) (1993) 99.
K. Lohrberg and E. Hillrich, DECHEMA Monogr. 125 (Elektrochem. Stoffgewinnung: Grundlagen Verfahreustech.), (1992) 221.
P. C. Foller, R. J. Allen, R. J. Vora, R. T. Bombard and M. DeMarinis, ‘The use of gas diffusion electrodes in the on-site generation of oxidants and reductants’, Fifth international forum on ‘Electrolysis in the Chemical Industry’, sponsored by Electrosynthesis Co. (10–14 Nov. 1991), Ft. Lauderdale, FA.
P. C. Foller, ‘The use of gas diffusion electrodes prospective industrial processes’, Electrochemical Processing: Innovation & Progress, Sponsored by ICI Chemicals and Polymers Ltd and EA Technology Ltd, Glasgow, UK (21–23 Apr. 1993).
P. C. Foller, ‘The On-Site Production of Hydrogen Peroxide in Caustic Soda’, Electric Power Research Institute Pulp and Paper Office, Project Advisory Council Meeting, IPST, Atlanta, GA, (7–8 Oct. 1993).
P. C. Foller, to be presented at the 187th meeting of the Electrochemical Society, Reno, NV, (21–26 May 1995).
M. Traube, Ber. Kgl. Akad. Wiss. Berlin (1887) 1041.
E. Berl, US Patents: (a) 2 000 815 (1935), (b) 2 091 129 (1937), (c) 2 091 130 (1937) and (d) 2 093 989 (1937).
E. Berl, Trans. Electrochem. Soc. 76 (1939) 359.
S. Mizuno, Denki Kagaku 17 (1949) 262.
S. Mizuno, ibid.Denki Kagaku 17 (1949) 288.
K. Otsuka and I. Yamanaka, Electrochim. Acta 35(2) (1990) 319.
A. S. Woodman, E. Anderson, E. J. Taylor and M. E. Fraser, PSI technical report 1271, PSI Technology Co., Andover, MA (Sept. 1993).
O. Spalek, J. Balej and K. Balogh, Coll. Czech. Chem. Comm. 42 (1977) 952.
O. Spalek, ibid.Coll. Czech. Chem. Comm. 42 (1977) 2747.
IdemO. Spalck, ibid.Coll. Czech. Chem. Comm. 44 (1979) 996.
J. Balej, ibid.Coll. Czech. Chem. Comm. 36 (1971) 426.
IdemJ. Balej, ibid.Coll. Czech. Chem. Comm. 37 (1972) 2830.
J. Balej, K. Balogh, O. Spalek, J. Vachuda and W. Havlicek, Czech. patent 175 863 (1979).
O. Spalek and J. Balej, Coll. Czech. Chem. Comm. 46 (1981) 2052.
J. Balej, K. Balogh, P. Stopka and O. Spalek, ibid.Coll. Czech. Chem. Comm. 45 (1980) 3249.
J. Divisek, K. Motlik, J. Vachuda and W. Havlicek, Czech. Patents 143 739 and 140 247 (1971).
D. H. Grangaard, US Patents: (a) 3 459 652 (1969), (b) 3529 997 (1970), (c) 3 591 470 (1971), (d) 3592749 (1971), (e) 3 607 687 (1971), and (f) 3 462 351 (1969).
H. Lemoyne and P. Duquet, European Patent 4 066 663 (1981).
T. Berzins and L. W. Gosser, US Patent 5 112 702 (1992).
F. J. Wolfgang and B. Kastening, US Patent 4 142 949 (1979).
S. Stucki, US Patent 4 455 203 (1984).
A. Porta, J.-M. Fresnel and A. Kulhanek, US Patent 4 350 575 (1982).
K. Halfar, M. L. Hitchman, and W. Mehl, US Patent 3 856 640 (1974).
B. Kastening and W. Faul, US Patent 3 968 273 (1976).
J. S. C. Chiang et al., US Patents: (a) 4 693 794 (1987), (b) 4731 173 (1988), (c) 4 753 718 (1988), (d) 4 758 317 (1988), and (c) 5 149 414 (1992).
J. Fenton and P. Tatapudi, J. Electrochem. Soc. 140(4) (1993) L55.
IdemJ. Fenton and P. Tatapudi, ibid.J. Electrochem. Soc. 141(5) (1994) 1174.
Zhiping Yuan et al., Zhongguo Zaozhi (China Pulp and Paper) 2(5) (1983) 2.
N. K. Khoreva et al., Zh. Prikladnoi Khimii 60(5) (1987) 1188.
V. A. Malyshev, O. E. Abolin and V. L. Kornienko, J. Appl. Chem., USSR 64(10) (1991) 2040.
H. Takenaka, Y. Kawami, I. Uehara, N. Wakabayashi and M. Motone, Denki Kagaku Oyobi Kogyo Butsuri. Kagaku 57(11) (1989) 1073.
N. Wakabayashi, H. Takenaka, Denki Kagaku 61(10) (1993) 1192.
C. W. Olomon (a-c) and A. P. Watkinson (a, b), US Patents: (a) 3 969 201 (1976), (b) 4 118 305 (1978) and (c) 4 728 409 (1988).
C. W. Olomon and A. P. Watkinson, J. Appl. Electrochem. 9 (1979) 117.
C. W. Olomon, J. Electrochem. Soc. 126 (1979) 1885.
Idem, ‘Electrochemical Synthesis and Separation Technology in the Paper and Pulp Industry’, Sixth international forum on ‘Electrolysis in the Chemical Industry’ Ft. Lauderdale, FL, (8–12 Nov. 1992).
C. W. Olomon, US Patent 5 074 957 (1991).
E. E. Kalu and C. W. Olomon, J. Appl. Electrochem. 20 (1990) 932.
J. A. McIntyre and R. F. Phillips, paper 399, 161st meeting of the Electrochemical Society, Montreal, Canada (9–14 May 1982).
G. Brown, D. F. Dong, J. A. McIntyre and R. F. Phillips, paper 11-5, TAPPI pulping conference, Houston, TX (24–26 Oct. 1983).
T. R. Bierschenk, US Patent 4 631 200 (1986).
J. A. McIntyre, US Patents: (a) 4 187 350 (1980), (b) 4224 129 (1980), (c) 4 260 469 (1981), (d) 4 317 704 (1982), (e) 4 341 606 (1982) (f) 4 406 758 (1983), (g) 4 431 494 (1984), (h) 4 457 953 (1984), (i) 4 481 303 (1984), and (j) 4 511 441 (1985).
D. F. Dong and A. L. Clifford, US Patents: (a) 4 891 107 (1990) and (b) 4 921 587 (1990).
D. F. Dong, E. Noonan, D. Rogers, A. Clifford, K. Benesch and R. Loftfield, US Patent 4 872 957 (1989).
I. Mathur, A. James and D. Bissett, US Patent 4 927 509 (1990).
D. J. Rogers, R. D. Klassen, A. James and I. Mathur, US Patent 4 969 981 (1990).
A. Clifford, D. F. Dong, E. Giziewicz and D. Rogers, paper 559, 177th meeting of the Electrochemical Society, Montreal, Canada, (6–11 May 1990).
Brochure and an economic analysis distributed by Dow Canada, Etobicoke, Ontario (June 1993).
Private communication, C. Thomas, Fort Howard paper.
R. J. Jasinski and C. G. Kuehn, US Patent 4 384 931 (1983).
C. G. Keuhn, F. Leder, R. J. Jasinski and K. Gaunt, J. Electrochem. Soc. 130(5) (1983) 1117.
C. G. Kuehn and F. Leder, US Patent 4 357 217 (1982).
J. B. Davison, US Patent 4 430 176 (1984).
M. Pourbaix, ‘Atlas of electrochemical potentials in aqueous solution’, Permagon Press, NY (1966), pp. 107.
R. W. Lindstrom, US Patent 4 647 359 (1987).
R. J. Allen, R. W. Lindstrom and W. Juda, US Patents: (a) 4248 682 (1981) and (b) 4 293 396 (1981).
K. Kordesch, US Patent 3899354 (1975).
D. F. Kroon and J. K. Dahms, ‘Fuel cell electrodes: Part 1’, Elsevier Sequoia Patent Reports, Elsevier Sequoia SA, Lausanne (1974).
D. F. Kroon and J. K. Dahms, ‘Fuel cell electrodes: Part 2’, Elsevier Sequoia Patent Reports, Elsevier Sequoia SA, Lausanne (1974).
R. Mosdale, M. Wakizoe and S. Srinivasan, paper 610, 185th meeting of the Electrochemical Society, San Francisco, CA, (22–27 May 1994).
J. Jorissen and K. H. Simmrock, J. Appl. Electrochem. 21 (1991) 869.
M. Paleologou and R. M. Barry, US Patent 5 006 211 (1991).
A. L. Clifford and D. J. Rogers, US Patents: (a) 5 106 464 (1992) and (b) 5 244 547 (1993).
W. C. Schumb, C. N. Satterfield and R. L. Wentworth, ‘Hydrogen peroxide’, ACS Monographs Series, Reinhold, New York, (1955) pp. 515–46.
C. W. Olomon, Canadian Patent 1 214 747 (1986).
J. Abbot and D. G. Brown, Can. J. Chem. 68(9) (1990) 1537.
FM21-SP Brochure, ICI Chemicals and Polymers, Ltd, Runcorn, Cheshire, UK (1993).
L. Gestaut, ‘Oxygen Electrodes for Energy Conversion and Storage’, Final Report, US Department of Energy Contracts DE-AC03-76SF00098 and DE-AC02-77ET25502 to Eltech Systems, Inc., Lawrence Berkeley Laboratory report 16679 (1983) pp. 371; M. Niksa, Electrode Corporation, private communication (1994).
J. A. McIntyre, Interface 4(1) (1995) 29.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Foller, P.C., Bombard, R.T. Processes for the production of mixtures of caustic soda and hydrogen peroxide via the reduction of oxygen. J Appl Electrochem 25, 613–627 (1995). https://doi.org/10.1007/BF00241923
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00241923