Abstract
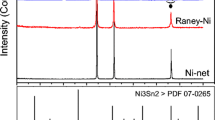
The hydrogen evolution reaction (h.e.r) was studied in alkaline solutions on two types of electrodes: (1) obtained by alloying Raney nickel without or with nickel and (ii) by pressing Raney nickel and nickel powders at room temperature. The obtained electrodes are usually very active for the h.e.r. The most active electrode was obtained by pressing Raney nickel with nickel powder (50 wt %). It was characterized by a large roughness factor, R ∼ 10 000 and a very low overpotential at the current density of 250 mA cm−2, η250 = 56 mV. The mechanism of the h.e.r. was studied using a.c. impedance measurements. The high electrode activity is connected with the increase in the intrinsic activity of the porous electrode surface.
Similar content being viewed by others
References
‘Electrochemical Hydrogen Technologies’, H. Wendt, (Ed.), Elsevier, Amsterdam (1990) p. 38.
B. V. Tilak, A. C. Ramamurthy and B. E. Conway, Proc. Indian Acad. Sci. (Chem. Sci.) 97 (1986) 359.
K. Lohrberg and P. Kohl, Electrochim. Acta 29 (1984) 1557.
E. Endoh, H. Otuma, T. Morimoto and Y. Oda, Int. J. Hydrogen Energy 7 (1987) 473.
Y. Choquette, H. Ménard and L. Brossard, ibid. 14 (1989) 637; ibid. 15 (1990) 21.
T. J. Gray, U.S. patent 4 240 895 (1980).
R. Heinne, M. v. Bradtke, W. Schnumberger and W. Weber, Proceedings of the 11th International Thermal Spraying Conference, ITSC, Montreal, 8–12 Sept. (1986) p. 61.
J. Divisek, H. Schmitz and J. Balej, J. Appl. Electrochem. 19 (1989) 519.
J. Divisek, P. Malinowski, J. Mergel and H. Schmitz, Int. J. Hydrogen Energy 13 (1988) 141.
J. Divisek, J. Mergel and H. Schmitz, ibid. 15 (1990) 105.
L. Chen and A. Lasia, J. Electrochem. Soc. 138 (1991) 3321.
C.R.S. Needs and N. Del, U.S. Patent 4 116 804 (1978).
A. Rami and A. Lasia, J. Appl. Electrochem. 22 (1992) 376.
M. B. F. Santos, E. P. da Silva, R. Andrade, Jr. and J. A. Dias, Electrochim. Acta 37 (1992) 29.
L. Chen and A. Lasia, J. Electrochem. Soc., 139 (1992) 3214.
A. Lasia and A. Rami, J. Electroanal. Chem. 294 (1990) 123.
Y. Choquette, A. Lasia, L. Brossard and H. Menard, J. Electrochem. Soc. 137 (1990) 1723.
W. H. Mulder, J. H. Sluyters, T. Pajkossy and L. Nyikos, J. Electoanal. Chem. 285 (1990) 103.
B. V. Tilak, S. Venkatesh and S. K. Rangarajan, J. Electrochem. Soc. 136 (1989) 1977.
Y. Choquette, L. Brossard, A. Lasia and H. Ménard, Electrochim. Acta 35 (1990) 1251.
P. Los, L. Brossard, H. Dumont, A. Lasia, J. Lessard and H. Ménard, Proceedings of the 5th Canadian Hydrogen Workshop, Ottawa, February, 1992, in press.
G. J. Brug, A. L. G. van der Eeden, M. Sluyters-Rehback and J. H. Sluyters, J. Electroanal. Chem. 176 (1984) 275.
P. Los and A. Lasia, J. Electroanal. Chem., 333 (1992) 115.
R. de Levie, J. Electroanal. Chem. 261 (1990) 1; 281 (1990) 1.
P. K. Wrona, A. Lasia, M. Lessard and H. Menard, Electrochim. Acta 37 (1992) 1283.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Los, P., Rami, A. & Lasia, A. Hydrogen evolution reaction on Ni-Al electrodes. J Appl Electrochem 23, 135–140 (1993). https://doi.org/10.1007/BF00246950
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00246950