Abstract
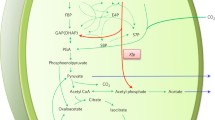
The cyanobacterium Microcystis PCC7806 fermented endogenously stored glycogen to ethanol, acetate, CO2, and H2 when incubated anaerobically in the dark. The switch from photoautotrophic to fermentative metabolism did not require de novo protein synthesis, and fermentation started immediately after cells had been transferred to dark anoxic conditions. From the molar ratios of the products and from enzyme activities in cell-free extracts, it was concluded that glucose derived from glycogen was degraded via the Embden-Meyerhof-Parnas pathway. In addition, CoA-dependent pyruvate:ferredoxin oxidoreductase, alcohol dehydrogenase, acetate kinase, and hydrogenase were present. The specific activities of these enzymes were sufficiently high to account for the rates of product formation by cell suspensions.
Similar content being viewed by others
References
Bergmeyer HE, Gawehn K, Grassl M (1974) Enzyme als biochemische Reagentien. In: Bergmeyer HE (ed) Methoden der enzymatischen Analyse, vol 1. Verlag Chemie, Weinheim, pp 454–558
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248–254
Bruinenberg PM, Van Dijken JP, Scheffers WA (1983) An enzymic analysis of NADPH production and consumption in Candida utilis. J Gen Microbiol 129: 965–971
Gottschalk G (1979) Bacterial metabolism. Springer, Berlin Heidelberg New York
Herbert D, Phipps PJ, Strange, RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 5B. Academic Press, London New York, pp 209–344
Heyer H, Stal LJ, Krumbein WE (1989) Simultaneous heterolactic and acetate fermentation in the marine cyanobacterium Oscillatoria limosa incubated anaerobically in the dark. Arch Microbiol 151: 558–564
Houchins JP (1984) The physiology and biochemistry of hydrogen metabolism in cyanobacteria. Biochim Biophys Acta 768: 227–255
Ibelings BW, Mur LR (1992) Microprofiles of photosynthesis and oxygen concentration in Microcystis scums. FEMS Microbiol Ecol 86: 195–203
Krogmann DW (1991) The low-potential cytochrome c of cyanobacteria and algae. Biochim Biophys Acta 1058: 35–37
Lipmann F, Tuttle LC (1945) A specific method for the determination of acyl phosphates. J Biol Chem 159: 21–28
Margheri MC, Allotta G (1993) Homoacetic fermentation in the cyanobacterium Nostoc sp. strain Cc from Cycas circinalis. FEMS Microbiol Letters 111: 213–218
Neuer G, Bothe H (1982) The pyruvate:ferredoxin oxidoreductase in heterocysts of the cyanobacterium Anabaena cylindrica. Biochim Biophys Acta 716: 358–365
Oren A, Shilo M (1979) Anaerobic heterotrophic dark metabolism in the cyanobacterium Oscillatoria limnetica: sulfur respiration and lactate fermentation. Arch Microbiol 122: 77–84
Revsbech NP, JØrgensen BB, Blackburn TH, Cohen Y (1983) Microelectrode studies of the photosynthesis and O2, H2S and pH profiles of a microbial mat. Limnol Oceanogr 28: 1062–1074
Reynolds CS, Walsby AE (1975) Waterblooms. Biol Rev 50: 437–481
Reynolds CS, Jaworski GHM, Cmiech HA, Leedale, GF (1981) On the annual cycle of the blue-green alga Microcystis aeruginosa Kütz Emend Elenkin. Philos Trans R Soc Lond Biol 293: 419–473
Richardson LL, Castenholz RW (1987) Enhanced survival of the cyanobacterium Oscillatoria terebriformis in darkness under anaerobic conditions. Appl Environ Microbiol 53: 2151–2158
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111: 1–61
Sanchez JJ, Palleroni NJ, Doudoroff, M (1975) Lactate dehydrogenases in cyanobacteria. Arch Microbiol 104: 57–65
Schäfer T, Schönheit P (1991) Pyruvate metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus. Acetate formation from acetyl-CoA and ATP synthesis are catalysed by an acetyl-CoA synthetase (ADP forming). Arch Microbiol 155: 366–377
Scherer S (1990) Do photosynthetic and respiratory electron transport chains share redox proteins? Trends Biochem Sci 15: 458–462
Schwartz ER, Reed LJ (1970) Regulation of the activity of the pyruvate dehydrogenase. Biochemistry 9: 1434–1439
Smith AJ (1982) Modes of cyanobacterial carbon metabolism. In: Carr NG, Whitton BA (eds) The biology of cyanobacteria. Blackwell, Oxford London, pp 47–85
Smith AJ (1983) Modes of cyanobacterial carbon metabolism. Ann Microbiol (Paris) 134 B: 93–113
Tombras Smith L, Kaplan NO (1980) Purification, properties and kinetic mechanism of coenzyme A-linked aldehyde dehydrogenase from Clostridium kluyveri. Arch Biochem Biophys 203: 663–675
Van der Oost J, Bulthuis BA, Feitz S, Krab K, Kraayenhof K (1989) Fermentation metabolism of the unicellular cyanobacterium Cyanothece PCC 7822. Arch Microbiol 152: 415–419
Van Dijken JP, Quayle JR (1977) Fructose metabolism in four Pseudomonas species. Arch Microbiol 114: 281–286
Van Liere L, Mur LR, Gibson CE, Herdman M (1979) Growth and physiology of Oscillatoria agardhii Gomont cultivated in continuous culture with a light-dark cycle. Arch Microbiol 123: 315–318
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moezelaar, R., Stal, L.J. Fermentation in the unicellular cyanobacterium Microcystis PCC7806. Arch. Microbiol. 162, 63–69 (1994). https://doi.org/10.1007/BF00264374
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00264374