Summary
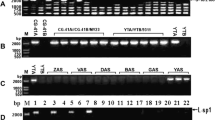
Variation in mitochondrial genome organization and expression between male fertile and sterile nuclear-cytoplasmic combinations of sorghum has been examined. Cytoplasmic genotypes were classified into eleven groups on the basis of restriction endonuclease digestion of mitochondrial DNA (mtDNA) and five groups on the basis of mitochondrial translation products. These cytoplasms were further characterized by hybridization of specific gene probes to Southern blots of EcoRI digested mtDNA, and identification of the fragment location of four mitochondrial genes. Variation was observed in the genomic location and copy number of the F1 ATPase α-subunit gene, as well as the genomic location and gene product of the cytochrome c oxidase subunit I gene. The effect of nuclear genotype on mitochondrial genome organization, expression and the presence of two linear plasmid-like mtDNA molecules was examined. Our results indicate that nuclear-mitochondrial interactions are required for regulation of mitochondrial gene expression. When a cytoplasm is transferred from its natural to a foreign nuclear background some changes in the products of in organello mitochondrial protein synthesis occur. In a number of cytoplasmic genotypes these changes correlate with the expression of cytoplasmic male sterile phenotype, suggesting a possible molecular basis for this mutation.
Similar content being viewed by others
References
Bailey-Serres J, Hanson DK, Fox T, Leaver CJ (1986) Mitochondrial genome rearrangement leads to extension and relocation of the cytochrome c oxidase subunit I gene in sorghum. Cell (in press)
Beckett JB (1971) Classification of male sterile cytoplasms in maize (Zea mays L.). Crop Sci 11:724–726
Borck KS, Walbot V (1982) Comparisons of restriction endonuclease patterns of mitochondrial DNA from normal and male sterile cytoplasms of Z. mays L. Genetics 102: 109–128
Boutry M, Farber AM, Charbonnier M, Briquet M (1984) Microanalysis of plant mitochondrial protein synthesis products. Plant Mol Biol 3:445–452
Conde MF, Pring DE, Schertz K, Ross W (1982) Correlation of mitochondrial DNA restriction endonuclease patterns with sterility expression in six male sterile sorghum cytoplasms. Crop Sci 22:536–539
Dawson A, Jones VP, Leaver CJ (1984) The apocytochrome b gene in maize mitochondria does not contain introns and is preceded by a potential ribosome binding site. EMBO J 3:207–2113
Dewey RE, Levings CS III, Timothy DH (1986) Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in Texas male-sterile cytoplasm. Cell 44:439–449
Dixon LK, Leaver CJ (1982) Mitochondrial gene expression and CMS in Sorghum. Plant Mol Biol 1:89–102
Dixon LK, Leaver CJ, Brettell RIS, Gengenbach BG (1982) Mitochondrial sensitivity to Drechslera maydis T-toxin and the synthesis of a variant mitochondrial polypeptide in plants derived from maize tissure cultures with Texas male-sterile cytoplasms. Theor Appl Genet 63:75–80
Dujardin G, Pajot P, Groudinsky O, Slonimski PP (1980) Long range control circuits within mitochondria and between nucleus and mitochondria. 1. Methodology and phenomenology of suppressors. Mol Gen Genet 179:469–482
Duvick DN (1965) Cytoplasmic pollen sterility in corn. Adv Genet 13:1–56
Edwardson JR (1970) Cytoplasmic male sterility. Bot Rev 36:341–420
Escote LJ, Gabay-Laughnan SJ, Laughnan JR (1986) Cytoplasmic reversions to fertility in CMS-S maize need not involve loss of linear mitochondrial plasmids. Plasmid 14:264–267
Forde BG, Oliver RJ, Leaver DJ (1978) Variation in mitochondrial translation products associated with male sterile cytoplasms in maize. Proc Natl Acad Sci USA 75: 3841–3845
Forde BG, Leaver CJ (1980) Nuclear and Cytoplasmic genes controlling synthesis of variant mitochondrial polypeptides in male-sterile maize. Proc Natl Acad Sci USA 77:418–422
Fox TD, Leaver CJ (1981) The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell 26:315–323
Groudinsky O, Dujardin G, Slonimski PP (1981) Long range control circuits within mitochondria and between nucleus and mitochondria. 2. Genetic and biochemical analyses of suppressors which selectively alleviate the mitochondrial intron mutations. Mol Gen Genet 184:493–503
Hanson M, Conde M (1985) Functioning and variation of cytoplasmic genomes: lessons from cytoplasmic-nuclear interactions affecting male fertility in plants. Int Rev Cytol 94:213–265
Isaac PG, Jones VP, Leaver CJ (1985 a) The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J 4:1617–1623
Isaac PG, Brennicke A, Dunbar SM, Leaver CJ (1985b) The mitochondrial genome of fertile maize (Zea mays L.) contains two copies of the gene encoding the α-subunit of the F1 ATPase. Curr Genet 10:321–328
Kemble RJ, Flavell RB, Brettell RIS (1983) Mitochondrial DNA analysis of fertile and sterile maize plants derived from tissue culture with the Texas male sterile cytoplasm. Theor Appl Genet 62:213–217
Kruszewska A, Szczesniak B (1985) Functional nuclear suppressor of mitochondrial oxi2 mutation in yeast. Curr Genet 10:87–93
Labouesse M, Dujardin G, Slonimski PP (1985) The yeast nuclear gene NAM2 is essential for mitochondrial DNA integrity and can cure a mitochondrial RNA-maturase deficiency. Cell 41:133–143
Laughnan J, Gabay-Laughnan S (1983) Cytoplasmic male sterility in maize. Annu Rev Genet 17:27–48
Leaver CJ, Hack E, Forde BG (1983) Protein synthesis by isolated plant mitochondria. Meth Enzymol 97:475–486
Leaver CJ, Gray M (1982) Mitochondrial genome organization and expression in higher plants. Annu Rev Plant Physiol 33:373–402
Leaver CJ, Isaac PG, Bailey-Serres J, Small ID, Hanson DK, Fox TD (1985) Recombination events associated with the cytochrome c oxidase subunit I gene in fertile and cytoplasmic male sterile maize and sorghum, vol 2. In: Quagliarello E, Slater EC, Palmieri F, Saccone C, Kroon AM (eds) Achievements and perspectives in mitochondrial biogenesis. Elsevier Science, Amsterdam, pp 111–122
Leroy P, Bazetoux FO (1985) A comparison between mitochondrial DNA of an isogenic male sterile (S) and male fertile (F) couple (HA89) of sunflower. Curr Genet 9: 243–251
Levings CS III, Pring DR (1977) Diversity of mitochondrial genomes among normal cytoplasms of maize. J Hered 68:350–354
Messing J (1983) New M13 vectors for cloning. Meth Enzymol 101:20–89
Pring DR, Conde MF, Scherte KF (1982) Organelle genome diversity in sorghum: male sterile cytoplasms. Crop Sci 22:414–421
Quinby JR (1980) Interactions of genes and cytoplasms in male sterility in sorghums. In: Proc 35th Corn Sorghum Res Conf. Am Seed Trade Assoc, Chicago Ill, pp 5–8
Rao J (1962) Occurance of cytoplasmic-genetic male-sterility in some Indian sorghums. Indian J Genet Plant Breed 22:257–259
Rigby PW, Dieckman M, Rhoades C, Berg P (1977) Labelling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol 113:237–251
Schardl CL, Lonsdale DM, Pring DR, Rose KR (1984) Linearization of maize mitochondrial chromosomes by recombination with linear episomes. Nature 310:292–296
Schertz K, Ritchey JM (1978) Cytoplasmic-genic male-sterility systems in sorghum. Crop Sci 18:890–893
Southern E (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Stephens JC, Holland RP (1954) Cytoplasmic male-sterility for hybrid sorghum seed production. Agron J 46:20–23
Webster OJ, Singh SP (1964) Breeding behavior and histological structure of a nondehiscent anther character in Sorghum vulgare Pres. Crop Sci 4:656–658
Author information
Authors and Affiliations
Additional information
Communicated by F.Salamini
Rights and permissions
About this article
Cite this article
Bailey-Serres, J., Dixon, L.K., Liddell, A.D. et al. Nuclear-mitochondrial interactions in cytoplasmic male-sterile sorghum. Theoret. Appl. Genetics 73, 252–260 (1986). https://doi.org/10.1007/BF00289282
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00289282