Abstract
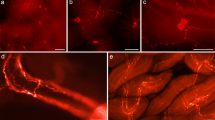
The distribution of catecholamines in the small and large intestine of flying foxes (Pteropus spp.) was investigated using glyoxylic-acid-induced fluorescence and immunohistochemical staining of tyrosine hydroxylase and dopamine-β-hydroxylase. Dense networks of varicose axons stained by each of these methods supplied blood vessels, the mucosa and both submucous and myenteric ganglia, but were scarce in the circular and longitudinal muscle. The majority (>90%) of submucous neuronal perikarya contained both enzymes and most of these also exhibited catecholamine fluorescence. Somata of similar staining characteristics were less common in the myenteric plexus, where single cells were found in only the minority of ganglia. All of the stained submucosal somata and mucosal axons contained vasoactive intestinal peptide, whereas catecholamine-containing axons that supplied the ganglia, external muscle and blood vessels did not. It is concluded that (1) there is dense catecholamine innervation of most tissues in the flyingfox intestine, similar to many other mammals, (2) mucosal axons originate from enteric catecholamine neurons, not found in other mammals, and (3) axons supplying the blood vessels and enteric ganglia are probably of sympathetic origin and can be distinguished from the intrinsic catecholamine-containing axons by their lack of vasoactive intestinal peptide. The roles and interactions of these two types of catecholamine innervation in the control of secretion and motility remain to be identified.
Similar content being viewed by others
References
Bennett MR, Burnstock G, Holman ME (1966) Transmission from perivascular inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol (Lond) 182:527–540
Bornstein JC, Furness JB (1988) Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst 25:1–13
Cooke HJ (1986) Neurobiology of the intestinal mucosa. Gastroenterology 90:57–81
Costa M, Gabella G (1971) Adrenergic innervation of the alimentary canal. Z Zellforsch 122:357–377
Costa M, Furness JB, Gabella G (1971) Catecholamine containing nerve cells in the mammalian myenteric plexus. Histochemie 25:103–106
Furness JB, Costa M (1971) Morphology and distribution of intrinsic adrenergic neurones in the proximal colon of the guinea-pig. Z Zellforsch 120:346–363
Furness JB, Costa M (1974) The adrenergic innervation of the gastrointestinal tract. Ergeb Physiol 69:1–51
Furness JB, Costa M (1987) The enteric nervous system. Churchill Livingstone, Edinburgh
Gillespie JS (1962) Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic nerve stimulation. J Physiol (Lond) 162:54–75
Keast JR (1987) Mucosal innervation and control of water and ion transport in the intestine. Rev Physiol Biochem Pharmacol 109:1–60
Keast JR (1991) Patterns of co-existence of peptides and differences of nerve fibre types associated with noradrenergic and non-noradrenergic (putative cholinergic) neurons in the major pelvic ganglion of the male rat. Cell Tissue Res 266:405–415
Kitahama K, Luppi P-H, Berod A, Goldstein M, Jouvet M (1987) Localization of tyrosine hydroxylase-immunoreactive neurons in the cata hypothalamus, with special reference to fluorescence histochemistry. J Comp Neurol 262:578–593
Llewellyn-Smith IJ, Furness JB, O'Brien PE, Costa M (1984) Noradrenergic nerves in human small intestine. Distribution and ultrastructure. Gastroenterology 87:513–529
Morris JL, Gibbins IL (1987) Neuronal colocalization of peptides, catecholamines, and catecholamine-synthesizing enzymes in guinea pig paracervical ganglia. J Neurosci 7:3117–3130
Skagerberg G, Meister B, Hökfelt T, Lindvall O, Goldstein M, Joh T, Cuello AC (1988) Studies on dopamine-, tyrosine hydroxylase-and aromatic L-amino acid decarboxylase-containing cells in the rat diencephalon: comparison between formaldehyde-induced histofluorescence and immunofluorescence. Neuroscience 24:605–620
Tedman RA, Hall LS (1985a) The absorptive surface area of the small intestine of Pteropus poliocephalus (Megachiroptera: Pteropodidae): an important factor in rapid food transit? Aust Mamm 8:271–278
Tedman RA, Hall LS (1985b) The morphology of the gastrointestinal tract and food transit in the fruit bats Pteropus alecto and P. poliocephalus (Megachiroptera). Aust J Zool 33:625–640
Torre JC de la (1980) An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods 3:1–5
Vizi ES, Knoll J (1971) The effects of sympathetic nerve stimulation and guanethidine on parasympathetic neuroeffector transmission: the inhibition of acetylcholine release. J Pharm Pharmacol 23:918–925
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Keast, J.R. Catecholamine innervation of the intestine of flying foxes (Pteropus spp.): a substantial supply from enteric neurons. Cell Tissue Res 276, 403–410 (1994). https://doi.org/10.1007/BF00306126
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00306126