Summary
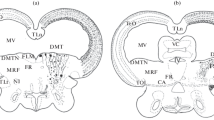
We have investigated the influence of meningeal cells on the development of the cerebellum by destroying these cells with 6-hydroxydopamine in hamsters of different ages. The ensuing foliation and lamination disruption in the cerebellar vermis is attributed to a disintegration of the cerebellar surface and a disorganization of the glial scaf-fold of the cerebellar cortex due to a loss of meningeal-glial interaction in stabilizing the extracellular matrix at the glia limitans superficialis (v. Knebel Doeberitz et al. 1986, Neuroscience 17:409–426). The severity of these cerebellar defects is correlated with the ontogenetic stage at which meningeal cells are destroyed, being greatest after treatment at postnatal day 1 and decreasing thereafter until day 5 and beyond, when no abnormalities occur, although all meningeal cells are destroyed throughout. The absence of cerebellar defects after destruction of meningeal cells at day 5 or later is associated firstly with the end of the period of branching morphogenesis of the cerebellum when all folial primordia are established, and, secondly, with the maturation of the glia limitans superficialis. These findings indicate that meningeal cells stabilize the cerebellar surface and glial scaffold over a critical period that ends, when the pattern of cerebellar foliation is established, and when the glia limitans superficialis has reached a mature state. Beyond this stage glial end-feet alone are sufficient to maintain the epithelial integrity of the cerebellum.
Similar content being viewed by others
References
Allen C, Sievers J, Berry M, Jenner S (1981) Experimental studies on cerebellar foliation. II. A morphometric analysis of cerebellar fissuration defects and growth retardation after neonatal treatment with 6-OHDA in the rat. J Comp Neurol 202:771–784
Altman J (1973) Experimental reorganization of the cerebellar cortex. III. Regeneration of the external germinal layer and granule cell ectopia. J Comp Neurol 149:153–180
Altman J (1982) Morphological development of the rat cerebellum and some of its mechanisms. Exp Brain Res [Suppl] 6:8–49
Altman J, Das GD (1966) Autoradiographic and histological studies of post-natal neurogenesis I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating 3H-thymidine in neonatal rats. With special reference to some brain regions. J Comp Neurol 126:337–390
Banerjee SD, Cohn RH, Bernfield MR (1977) Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol 73:445–463
Basco E, Hajos F, Fülop Z (1977) Proliferation of Bergmann glia in the developing rat cerebellum. Anat Embryol 151:219–222
Bejahr A, Roujansky P, de Barry J, Gombos G (1985) Different effect of methylazoxymethanol on mouse cerebellar development depending on the age of injection. Exp Brain Res 57:279–285
Bernfield MR (1970) Collagen synthesis during epitheliomesenchymal interactions. Dev Biol 28:191–201
Bernfield MR, Banerjee SD (1978) The basal lamina in epithelial-mesenchymal morphogenetic interactions. In: Kefalides NA (ed) Biology and chemistry of basement membranes. Academic Press, New York, pp 137–148
Bernfield MR, Banerjee SD (1982) The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev Biol 9:291–305
Bernfield MR, Banerjee SD, Koda JE, Rapraeger AC (1984) Remodeling of the basement membrane as a mechanism of morphogenetic tissue interaction. In: Trelstad R (ed) The role of extracellular matrix in development. Liss, New York, pp 545–572
Ebels EJ (1970) The influence of age upon the effect of early postnatal X-irradiation on the development of the cerebellar cortex in rats. Acta Neuropathol 15:298–307
Ebels EJ (1972) Studies on ectopic granule cells in the cerebellar cortex — with a hypothesis as to their aetiology and pathogenesis. Acta Neuropathol 21:117–127
Grobstein C, Cohen J (1965) Collagenase: effect on the morphogenesis of embryonic salivary epithelium in vitro. Science 150:626–628
Hausmann B, Sievers J (1985) Cerebellar external granule cells are attached to the basal lamina from the onset of migration up to the end of their proliferative activity. J Comp Neurol 241:50–62
Kühl U, Timpl R, v d Mark K (1982) Synthesis of type IV collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Dev Biol 93:344–354
Kühl U, Öchalan M, Timpl R, Mayne R, Hay E, v d Mark K (1984) Role of muscle fibroblasts in the deposition of type IV collagen in the basal lamina of myotubes. Differentiation 28:164–172
Larsell O (1952) The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol 97:281–356
Lewis PD, Fülop Z, Hajos F, Balazs R, Woodhams PL (1977) Neuroglia in the internal germinal layer of the developing rat cerebellar cortex. Neuropathol Appl Neurobiol 3:183–190
Mangold U, Sievers J, Berry M (1984) 6-OHDA induced ectopia of external granule cells in the subarachnoid space covering the cerebellum. II. Differentiation of granule cells: a scanning and transmission electron microscopic study. J Comp Neurol 227:267–284
Nathanson N, Cole GA, van der Loos H (1969) Heterotopic cerebellar granule cells following administration of cyclophosphamide to suckling rats. Brain Res 15:532–536
Oster-Granite ML, Herndon RM (1976) The development of the cerebellar cortex of the Syrian hamster, Mesocricetus auratus. Foliation, cytoarchitecture, Golgi and electron microscopic studies. J Comp Neurol 163:443–480
Pehlemann FW, Sievers J, Berry M (1985) Meningeal cells are involved in foliation, lamination and neurogenesis of the cerebellum: evidence from 6-hydroxydopamine-induced destruction of meningeal cells. Dev Biol 110:136–146
Pehlemann FW, Mohr S, Korr H, Sievers J (1986) The influence of meningeal cells on cell proliferation in the cerebellum. In: Wolff JR, Sievers J, Berry M (eds) Mesenchymal-epithelial interactions in neural development. Springer, Berlin Heidelberg New York, in press
Rakic P (1971) Neuron-glia relationship during granule cell migration in developing cerebellar cortex: a Golgi and electron microscopic study in Macacus rhesus. J Comp Neurol 141:283–312
Rakic P (1975) Cell migration and neuronal ectopias in the brain. Birth Defects. 11:95–129
Rakic P (1982) The role of neuronal-glial cell interaction during brain development. In: Sears TA (ed) Neuronal-glial cell inter-relationships. Springer, Berlin Heidelberg New York, pp 25–38
Shiga T, Ichikawa M, Hirata J (1983) Spatial and temporal pattern of postnatal proliferation of Bergmann glial cells in rat cerebellum. An autoradiographic study. Anat Embryol 167:203–211
Shimada M, Langman J (1970a) Repair of the external granular layer after post-natal treatment with 5-fluorodeoxyuridine. Am J Anat 129:247–280
Shimada M, Langman J (1970b) Repair of the external granular layer of the hamster cerebellum after prenatal and post-natal administration of methylazoxymethanol. Teratology 3:119–134
Sievers J, Mangold U, Berry M, Allen C, Schlossberger HG (1981) Experimental studies on cerebellar foliation. I. A qualitative morphological analysis of cerebellar fissuration defects after neonatal treatment with 6-OHDA in the rat. J Comp Neurol 203:751–769
Sievers J, Mangold U, Berry M (1983a) 6-OHDA-induced ectopia of external granule cells in the subarachnoid space covering the cerebellum. Genesis and topography. Cell Tissue Res 230:308–336
Sievers J, Mangold U, Berry M (1983b) Die Beteiligung der Fibroblasten der Leptomeninx an der Morphogenese des Cerebellum: eine Hypothese. Verh Anat Ges 77:479–480
Sievers J, Pehlemann FW, Baumgarten HG, Berry M (1985) Selective destruction of meningeal cells by 6-hydroxydopamine: a tool to study meningeal-neuroepithelial interaction in brain development. Dev Biol 110:127–135
Sievers J, Pehlemann FW, Berry M (1986) Influences of meningeal cells on brain development: findings and hypothesis. Naturwissenschaften 73:188–194
Spooner BR, Faubion JM (1980) Collagen involvement in branching morphogenesis of embryonic lung and salivary gland. Dev Biol 77:84–102
Thompson HA, Spooner BS (1982) Inhibition of branching morphogenesis and alteration of glycosaminoglycan biosynthesis in salivary glands treated with β-D-xyloside. Dev Biol 89:417–424
Thompson HA, Spooner BS (1983) Proteoglycan and glycosaminoglycan synthesis in embryonic mouse salivary glands: effects of β-D-xyloside, an inhibitor of branching morphogenesis. J Cell Biol 96:1443–1450
von Knebel Doeberitz Ch, Sievers J, Sadler M, Pehlemann FW, Berry M, Halliwell P (1986) Destruction of meningeal cells over the newbron hamster cerebellum with 6-hydroxydopamine prevents foliation and lamination in the rostral cerebellum. Neuroscience 17:409–426
Wessels NK, Cohen JA (1968) Effects of collagenase on developing epithelia in vitro: lung, ureteric bud, and pancreas. Dev Biol 18:294–309
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sievers, J., Doeberitz, C.v.K., Pehlemann, FW. et al. Meningeal cells influence cerebellar development over a critical period. Anat Embryol 175, 91–100 (1986). https://doi.org/10.1007/BF00315459
Issue Date:
DOI: https://doi.org/10.1007/BF00315459