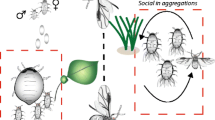
Abstract
Aphidiid parasitoids (Hymenoptera: Aphidiidae) of aphids generally exploit only a small percentage of the available host resources in the field. This limited impact on aphid populations has often been explained as a consequence of hyperparasitism. We propose that a wasp's reproductive strategy, as opposed to hyperparasitism, is the dominant factor in aphidiid population dynamics. A wasp's foraging efficiency and oviposition decisions are influenced by several variables, including searching behaviour between and within patches, host choice (as modified by the aphids' defensive behaviours), and plant structural complexity. Two broadly different patterns of host exploitation have evolved in aphidiid wasps in relation to ant-aphid mutualism. Firstly, in species that are exposed to predation and hyperparasitism, a female may leave a patch before all suitable hosts are parasitized. Because predators and hyperparasitoids tend to aggregate at high aphid or aphidiid densities, or in response to aphid honeydew, this strategy enables females to reduce offspring mortality by “spreading the risk” over several host patches. Secondly, in species that have evolved mechanisms to avoid aggression by mutualistic ants, females are able to exploit a hyperparasitoid-free resource space. Such species may concentrate their eggs in only a few aphid colonies, which are thus heavily exploited. Although hyperparasitism of species in the first group tends to reach high levels, its overall impact on aphid-aphidiid population dynamics is probably limited by the low average fecundity of most hyperparasitoids. We discuss the foraging patterns of aphidiid wasps in relation to aphid population regulation in general, and to classical biological control in particular. We argue that a parasitoid's potential to regulate the host population is largely determined by its foraging strategy. In an exotic parasitoid, a behavioural syndrome that has evolved and presumably is adaptive in a more diverse (native) environment may, in a more uniform (managed) environment, result in suboptimal patch-leaving and oviposition decisions, and possibly increased resource usage.
Similar content being viewed by others
References
Andow DA, Prokrym DR (1990) Plant structural complexity and host-finding by a parasitoid. Oecologia 82:162–165
Ayal Y, Green RF (1993) Optimal egg distribution among host patches for parasitoids subject to attack by hyperparasitoids. Am Nat 141:120–138
Beddington JR, Hammond PS (1977) On the dynamics of host-parasite-hyperparasite interactions. J Anim Ecol 46:811–821
Bennett AW, Sullivan DJ (1978) Defensive behavior against tertiary parasitism by the larva of Dendrocerus carpenteri, an aphid hyperparasitoid. J N Y Entomol Soc 86:153–160
Bocchino FJ, Sullivan DJ (1981) Effects of venoms from two aphid hyperparasitoids, Asaphes lucens and Dendrocerus carpenteri (Hymenoptera: Pteromalidae and Megaspilidae), on larvae of Aphidius smithi (Hymenoptera: Aphidiidae). Can Entomol 113:887–889
Boer PJ den (1968) Spreading of risk and stabilization of animal numbers. Acta Biotheor 18:165–194
Bosch R van den, Schlinger EI, Lagace CF, Hall J (1966) Parasitization of Acyrthosiphon pisum by Aphidius smithi a density dependent process in nature (Homoptera: Aphididae) (Hymenoptera: Aphidiidae). Ecology 47:1049–1055
Bosch R van den, Hom R, Matteson P, Frazer BD, Messenger PS, Davis CS (1979) Biological control of the walnut aphid in California: impact of the parasite, Trioxys pallidus. Hilgardia 47:1–13
Brodeur J, McNeil JN (1991) The effect of host plant architecture on the distribution and survival of Aphidius nigripes (Hymenoptera: Aphidiidae). Redia 74 (3) Appendix: 251–258
Brodeur J, McNeil JN (1992) Host behaviour modification by the endoparasitoid Aphidius nigripes: a strategy to reduce hyperparasitism. Ecol Entomol 17:97–104
Campbell A (1974) Seasonal changes in abundance of the pea aphid and its associated parasites in the southern interior of British Columbia. Ph. D. thesis, Simon Fraser University, Burnaby, BC, Canada
Campbell A, Mackauer M (1975) The effect of parasitism by Aphidius smithi (Hymenoptera: Aphidiidae) on reproduction and population growth of the pea aphid (Homoptera: Aphididae). Can Entomol 107:919–926
Campbell A, Frazer BD, Gilbert N, Gutierrez AP, Mackauer M (1974) Temperature requirements of some aphids and their parasites. J Appl Ecol 11:431–438
Carver M (1989) Biological control of aphids. In: Minks AK, Harrewijn P (eds) Aphids: their biology, natural enemies and control. (World crop pests, vol 2C) Elsevier, Amsterdam, pp 141–165
Charnov EL (1976) Optimal foraging: the marginal value theorem. Theor Popul Biol 9:129–136
Chesson PL, Murdoch WW (1986) Aggregation of risk: relationships among host-parasitoid models. Am Nat 127:696–715
Chow A, Mackauer M (1991) Patterns of host selection by four species of aphidiid (Hymenoptera) parasitoids: influence of host switching. Ecol Entomol 16:403–410
Cloutier C, Lévesque CA, Eaves DM, Mackauer M (1991) Maternal adjustment of sex ratio in response to host size in the aphid parasitoid Ephedrus californicus. Can J Zool 69: 1489–1495
Cohen MB, Mackauer M (1986) Lupine aphid, Macrosiphum albifrons (Homoptera: Aphididae): distribution and hymenopterous parasites in British Columbia. Environ Entomol 15:719–722
Cohen MB, Mackauer M (1987) Intrinsic rate of increase and temperature coefficients of the aphid parasite Ephedrus californicus (Hymenoptera: Aphidiidae). Can Entomol 119: 231–237
Collins MD, Ward SA, Dixon AFG (1981) Handling time and the functional response of Aphelinus thomsoni, a predator and parasite of the aphid Drepanosiphum platanoidis. J Anim Ecol 50:479–487
Cook RM, Hubbard SF (1977) Adaptive search strategies in insect parasites. J Anim Ecol 46: 115–125
Dean GJ, Jones MG, Powell W (1981) The relative abundance of the hymenopterous parasites attacking Metopolophium dirhodum (Walker) and Macrosiphum avenae (F.) (Hem., Aphididae). Bull Entomol Res 71: 307–315
Dempster JP (1983) The natural control of subpopulations of butterflies and moths. Biol Rev 58:461–481
Dransfield RD (1979) Aspects of host-parasitoid interactions of two aphid parasitoids, Aphidius urticae (Haliday) and Aphidius uzbeckistanicus (Luzhetski) (Hymenoptera, Aphidiidae). Ecol Entomol 4: 307–316
Farrell JA, Stufkens MW (1990) The impact of Aphidius rhopalosiphi (Hymenoptera: Aphidiidae) on populations of the rose grain aphid, Metopolophium dirhodum (Hemiptera: Aphididae) on cereals in Canterbury, New Zealand. Bull Entomol Res 80:377–383
Force DC, Messenger PS (1964) Fecundity, reproductive rates, and innate capacity for increase of three parasites of Therioaphis maculata (Buckton). Ecology 45:706–715
Frazer BD, Bosch R van den (1973) Biological control of the walnut aphid in California: the interrelationship of the aphid and its parasite. Environ Entomol 2:561–568
Freeland WJ (1986) Arms races and covenants: the evolution of parasite communities. In: Kikkawa J, Anderson DJ (eds) Community ecology: pattern and process. Blackwell, London, pp 289–308
Futuyma DJ, Slatkin M (eds) (1983) Coevolution. Sinauer, Sunderland, Mass
Gardner SM, Dixon AFG (1985) Plant structure and the foraging success of Aphidius rhopalosiphi (Hymenoptera: Aphidiidae). Ecol Entomol 10:171–179
Gerling DH, Roitberg BD, Mackauer M (1990) Instar-specific defense of the pea aphid, Acyrthosiphon pisum: influence on oviposition success of the parasite Aphelinus asychis (Hymenoptera: Aphelinidae). J Insect Behav 3:501–514
Gilbert N, Gutierrez AP (1973) A plant-aphid-parasite relationship. J Anim Ecol 42:323–340
Green RF (1984) Stopping rules for optimal foragers. Am Nat 123:30–40
Hafez M (1961) Seasonal fluctuations of population density of the cabbage aphid, Brevicoryne brassicae (L.) in the Netherlands, and the role of its parasite, Aphidius (Diaeretiella) rapae (Curtis). Tijdschr Plantenziekt 67:445–548
Hagen KS, Bosch R van den (1968) Impact of pathogens, parasites, and predators on aphids. Annu Rev Entomol 13:325–384
Hassell MP (1978) The dynamics of arthropod predator-prey systems. Monographs in population biology, 13. Princeton University Press, Princeton, NJ
Hassell MP (1985) Insect natural enemies as regulating factors. J Anim Ecol 54:323–334
Hassell MP (1986) Parasitoids and population regulation. In: Waage J, Greathead D (eds) Insect parasitoids. Academic Press, London, pp 201–224
Hassell MP, Waage JK (1984) Host-parasitoid population interactions. Annu Rev Entomol 29:89–114
Hawkins BA (1992) Parasitoid-host food webs and donor control. Oikos 65:159–162
Henter H (1992) Genetic variation within a wasp population in the ability to parasitize resistant aphid hosts. IX International Entomophagous Insect Workshop, 3–7 May 1992, Hawk's Cay, Florida
Höller C (1991) Movement away from the feeding site in parasitized aphids: host suicide or an attempt by the parasitoid to escape hyperparasitism? In: Polgár L, Chambers RJ, Dixon AFG, Hodek I (eds) Behaviour and impact of Aphidophaga. SPB, The Hague, pp 45–49
Hofsvang T (1991) Fecundity of aphid parasitoids in the family Aphidiidae (Hymenoptera). In: Polgár L, Chambers RJ, Dixon AFG, Hodek I (eds) Behaviour and impact of Aphidophaga. SPB, The Hague, pp 41–44
Horn DJ (1984) Vegetational complexity and parasitism of green peach aphids (Myzus persicae (Sulzer) (Homoptera: Aphididae)) on collards. J N Y Entomol Soc 92:19–26
Horn DJ (1989) Secondary parasitism and population dynamics of aphid parasitoids (Hymenoptera: Aphidiidae). J Kans Entomol Soc 62:203–210
Houston AI, McNamara JM (1986) The influence of mortality on the behaviour that maximizes reproductive success in a patchy environment. Oikos 47:267–274
Huffaker CB, Simmonds FJ, Laing JE (1976) The theoretical and empirical basis of biological control. In: Huffaker CB, Messenger PS (eds) Theory and practice of biological control. Academic Press, New York, pp 41–78
Hughes RD (1989) Biological control in the open field. In: Minks AK, Harrewijn P (eds) Aphids: their biology, natural enemies and control. (World crop pests, vol 2C) Elsevier, Amsterdam, pp 167–198
Hughes RD, Woolcock LT, Roberts JA, Hughes MA (1987) Biological control of the spotted alfalfa aphid, Therioaphis trifolii f. maculata, on lucerne crops in Australia by the introduced parasitic hymenopteran Trioxys complanatus. J Appl Ecol 24: 515–537
Hughes RD, Woolcock LT, Hughes MA (1992) Laboratory evaluation of parasitic hymenoptera used in attempts to biologically control aphid pests of crops in Australia. Entomol Exp Appl 63:177–185
Kambhampati S, Mackauer M (1989) Multivariate assessment of inter- and intraspecific variation in performance criteria of several pea aphid parasites (Hymenoptera: Aphidiidae). Ann Entomol Soc Am 82:314–324
Kfir R, Kirsten F (1991) Seasonal abundance of Cinara cronartii (Homoptera: Aphididae) and the effect of an introduced parasite, Pauesia sp. (Hymenoptera: Aphidiidae). J Econ Entomol 84:76–82
Klingauf F (1967) Abwebr- und Meidereaktionen von Blattläusen (Aphididae) bei Bedrohung durch Räuber und Parasiten. Z Angew Entomol 60:269–317
Kouamé KL, Mackauer M (1991) Influence of aphid size, age and behaviour on host choice by the parasitoid wasp Ephedrus californicus: a test of host-size models. Oecologia 88:197–203
Levine L, Sullivan DJ (1983) Intraspecific tertiary parasitoidism in Asaphes lucens (Hymenoptera: Pteromalidae), an aphid hyperparasitoid. Can Entomol 115:1653–1658
Li C, Roitberg BD, Mackauer M (1992) The search pattern of a parasitoid wasp, Aphelinus asychis, for its host. Oikos 65:207–212
Li C, Roitberg BD, Mackauer M (1993) Patch residence time and parasitism of Aphelinus asychis: a simulation model. Ecol Model (in press)
Lincicome DR (1971) The goodness of parasitism: a new hypothesis. In: Cheng TC (ed) Aspects of the biology of symbiosis. University Park Press, Baltimore, pp 139–227
Liu SS (1985) Aspects of the numerical and functional responses of the aphid parasite, Aphidius sonchi, in the laboratory. Entomol Exp Appl 37:247–256
Liu SS, Morton R, Hughes RD (1984) Oviposition preferences of a hymenopterous parasite for certain instars of its aphid host. Entomol Exp Appl 35:249–254
Lopez ER, Van Driesche RG, Elkinton JS (1990) Rates of parasitism by Diaeretiella rapae (Hymenoptera: Braconidae) for cabbage aphids (Homoptera: Aphididae) in and outside of colonies: Why do they differ? J Kans Entomol Soc 63:158–165
Luck R, Messenger PS, Barbieri JF (1981) The influence of hyperparasitism on the performance of biological control agents. In: Rosen D (ed) The role of hyperparasitism in biological control: a symposium. Division of Agricultural Sciences, Univ Calif Publ 4103:34–42
Mackauer M (1965) Parasitological data as an aid in aphid classification. Can Entomol 97:1016–1024
Mackauer M (1973) Host selection and host suitability in Aphidius smithi (Hymenoptera: Aphidiidae). In: Lowe AD (ed) Perspectives in aphid biology. Bull Entomol Soc N Z 2: 20–29
Mackauer M (1983) Quantitative assessment of Aphidius smithi (Hymenoptera: Aphidiidae): fecundity, intrinsic rate of increase, and functional response. Can Entomol 115:399–415
Mackauer M (1990) Host discrimination and larval competition in solitary endoparasitoids. In: Mackauer M, Ehler LE, Roland J (eds) Critical issues in biological control. Intercept, Andover, Hants, pp 41–62
Mackauer M, Chow FJ (1986) Parasites and parasite impact on aphid populations. In: McLean GD, Garret RG, Ruesink WG (eds) Plant virus epidemics: monitoring, modelling and predicting outbreaks. Academic Press, Sydney, pp 95–117
Mackauer M, Chow FJ (1990) The effect of stinging: aphidiid parasitoids (Hymenoptera) “prefer” pseudoparasitized pea aphids. Mitt Schweiz Entomol Ges 63:309–315
Mackauer M, Kambhampati S (1988) Parasitism of aphid embryos by Aphidius smithi: some effects of extremely small host size. Entomol Exp Appl 49:167–173
Mackauer M, Sequeira R (1993) Patterns of development in insect parasites. In: Beckage ME, Thompson SN, Federici BA (eds) Parasites and pathogens of insects, vol 1. Academic Press, Orlando, pp 1–23
Mackauer M, Starý P (1967) World Aphidiidae (Hym. Ichneumonoidea). Le François, Paris
Mangel M (1990) Dynamic information in uncertain and changing worlds. J Theor Biol 146:317–332
Matejko I, Sullivan DJ (1979) Bionomics and behavior of Alloxysta megourae, an aphid hyperparasitoid (Hymenoptera: Cynipidae). J N Y Entomol Soc 87:275–282
Matejko I, Sullivan DJ (1984) Interspecific tertiary parasitoidism between two aphid hyperparasitoids: Dendrocerus carpenteri and Alloxysta megourae (Hymenoptera: Megaspilidae and Cynipidae). J Wash Acad Sci 74:31–38
May RM, Hassell MP (1988) Population dynamics and biological control. In: Wood RKS, Way MJ (eds) Biological control of pests, pathogens and weeds: developments and prospects. Royal Society, London, pp 19–57
Messenger PS (1970) Bioclimatic inputs to biological control and pest management programs. In: Rabb RL, Guthrie FE (eds) Concepts of pest management. North Carolina State University, Raleigh, NC, pp 84–102
Mesterton-Gibbons M (1988) On the optimal compromise for a dispersing parasitoid. J Math Biol 26:375–385
Michalakis Y, Olivieri I, Renaud F, Raymond M (1992) Pleiotropic action of parasites: how to be good for the host. Trends Ecol Evol 7:59–62
Murdoch WM (1990) The relevance of pest-enemy models to biological control. In: Mackauer M, Ehler LE, Roland J (eds) Critical issues in biological control. Intercept, Andover, Hants, pp 1–24
Nault LR, Montgomery ME, Bowers WS (1976) Ant-aphid association: role of aphid alarm pheromones. Science 192:1349–1351
Nowierski RM (1979) The field ecology of the walnut aphid, Chromaphis juglandicola (Homoptera: Aphididae) and its introduced parasite, Trioxys pallidus (Hymenoptera: Aphidiidae): a qualitative and quantitative assessment of population regulation. Ph. D. thesis, University of California, Berkeley
Paetzold G, Vater D (1967) Populationsdynamische Untersuchungen an den Parasiten und Hyperparasiten von Brevicoryne brassicae (L.) (Homoptera, Aphididae). Acta Entomol Bohemoslov 64:83–90
Paetzold G, Vater D (1969) Untersuchungen zum Einfluß der Hyperparasiten auf die Populationsdynamik von Diaeretiella rapea (M'Intosh) (Hymenoptera: Aphidiidae). Ber 10 Wanderver Deutsch Entomol, Dresden 1965, 80:365–375
Pimentel D (1968) Population regulation and genetic feedback. Science 159:1432–1437
Price PW, Westoby M, Rice B (1988) Parasite-mediated competition: some predictions and tests. Am Nat 131:544–555
Roitberg BD (1990) Variation in behaviour of individual parasitic insects: bane or boon? In: Mackauer M, Ehler LE, Roland J (eds) Critical issues in biological control. Intercept, Andover, Hants, pp 25–39
Sequeira R, Mackauer M (1987) Host instar preference of the aphid parasite Praon pequodorum (Hymenoptera: Aphidiidae). Entomol Gen 12:259–265
Sequeira R, Mackauer M (1992) Nutritional ecology of an insect host-parasitoid association: the pea aphid — Aphidius ervi system. Ecology 73:183–189
Shirota Y, Carter N, Rabbinge R, Ankersmit GW (1983) Biology of Aphidius rhopalosiphi, a parasitoid of cereal aphids. Entomol Exp Appl 34:27–34
Singh R, Srivastava PN (1989) Life-table studies of an aphid hyperparasitoid Alloxysta pleuralis (Cameron) (Hymenoptera: Alloxystidae). J Appl Entomol 107:351–356
Slobodkin LB (1968) Toward a predictive theory of evolution. In: Lewontin RC (ed) Population biology and evolution. Syracuse University Press, Syracuse, NY, pp 187–205
Stadler B (1989) Untersuchungen zur Populationsökologie von Uroleucon jaceae (L.) (Homoptera, Aphididae) an Centaureen in Oberfranken. Diplomarbeit, University of Bayreuth
Stadler B, Völkl W (1991) Foraging patterns of two aphid parasitoids, Lysiphlebus testaceipes and Aphidius colemani on banana. Entomol Exp Appl 58:221–229
Starý P (1970) Biology of aphid parasites. Junk, The Hague
Starý P (1988) Aphidiidae. In: Minks AK, Harrewijn P (eds) Aphids: their biology, natural enemies and control. (World crop pests, vol 2B) Elsevier, Amsterdam, pp 171–184
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton, NJ
Stiling PD (1987) The frequency of density-dependence in insect host-parasitoid systems. Ecology 68:844–856
Stiling PD (1988) Density dependent processes and key factors in animal populations. J Anim Ecol 57:581–593
Strong DJ (1988) Parasitoid theory: from aggregation to dispersal. Trends Ecol Evol 3:277–280
Sullivan DJ (1987) Insect hyperparasitism. Annu Rev Entomol 32:49–70
Sullivan DJ (1988) Hyperparasites. In: Minks AK, Harrewijn P (eds) Aphids: their biology, natural enemies and control. (World crop pest, vol 2B) Elsevier, Amsterdam, pp 189–203
Sullivan DJ, Bosch R van den (1971) Field ecology of the primary parasites and hyperparasites of the potato aphid, Macrosiphum euphorbiae, in the east San Francisco Bay area. Ann Entomol Soc Am 64:389–394
Turchin P, Kareiva P (1989) Aggregation in Aphis varians: an effective strategy for reducing predation risk. Ecology 70:1008–1016
Vickerman GP, Wratten SD (1979) The biology and pest status of cereal aphids (Hemiptera: Aphididae) in Europe: a review. Bull Entomol Res 69:1–32
Völkl W (1990) Fortpflanzungsstrategien bei Blattlausparasitoiden: Konsequenzen ihrer Interaktionen mit Wirten und Ameisen. Ph.D. thesis, University of Bayreuth
Völkl W (1991) Species-specific larval instar preferences and aphid defense behaviour in three parasitoids of Aphis fabae. In: Polgár L, Chambers RJ, Dixon AFG, Hodek I (eds) Behaviour and impact of Aphidophaga. SPB, The Hague, pp 73–78
Völkl W (1992) Aphids or their parasitoids: who actually benefits from ant-attendance? J Anim Ecol 61:273–281
Völkl W, Mackauer M (1993) Interactions between ants attending Aphis fabae spp. cirsiiacanthoidis on thistles and foraging parasitoid wasps. J Insect Behav 6:301–312
Waage JK (1979) Foraging for patchily distributed hosts by the parasitoid, Nemeritis canescens. J Anim Ecol 48:353–371
Waage JK, Hassell MP (1982) Parasitoids as biological control agents — a fundamental approach. Parasitology 84:241–268
Walde SJ, Murdoch WW (1988) Spatial density dependence in parasitoids. Annu Rev Entomol 33:441–466
Walker GP, Cameron PJ (1981) The biology of Dendrocerus carpenteri (Hymenoptera: Ceraphronidae), a parasite of Aphidius species, and field observations of Dendrocerus species as hyperparasites of Acyrthosiphon species. N Z J Zool 8:531–538
Weißer W (1991) Das Eiablageverhalten von Blattlausparasitoiden (Hymenoptera: Aphidiidae): Welchen Einfluß haben Habitatfaktoren und das Parasitoidenalter? Diplomarbeit, University of Bayreuth
Wilson CG, Swincer DE (1984) Hyperparasitism of Therioaphis trifolii f. maculata (Homoptera: Aphididae) in South Australia. J Aust Entomol Soc 23:47–50
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mackauer, M., Völkl, W. Regulation of aphid populations by aphidiid wasps: does parasitoid foraging behaviour or hyperparasitism limit impact?. Oecologia 94, 339–350 (1993). https://doi.org/10.1007/BF00317107
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317107