Abstract
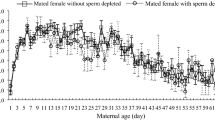
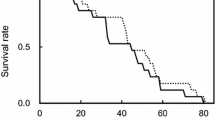
Maternal age influences offspring quality of many species of insects. This observed maternal age influence on offspring performance may be mediated through maternal age effects on egg size, which in turn may be directly influenced by the female's nutritional state. Thus, behaviors that influence a female's nutritional status will indirectly influence egg size, and possibly offspring life histories. Because males provide nutrients to females in their ejaculate, female mating frequency is one behavior which may influence her nutritional status, and thus the size of her eggs and the performance of her offspring. In this paper, I first quantify the influences of maternal age on egg size and offspring performance of the bruchid beetle, Callosobruchus maculatus. I then examine whether nutrients transferred during copulation reduce the magnitude of maternal age effects on egg size and larval performance when mothers are nutrient-stressed. Egg size and egg hatchability decreased, and development time increased, with increasing maternal age. Multiple mating and adult feeding by females both resulted in increased egg size. This increase in egg size of females mated multiply did not translate into reduced development time or increased body size and egg hatchability, but did correlate with improved survivorship of offspring produced by old mothers. Thus, it appears that because the influence of mating frequency on egg size is small relative to the influence of maternal age, the influence of nutrients derived from multiple mating on offspring life history is almost undetectable (detected only as a small influence on survivorship). For C. maculatus, female multiple mating has been demonstrated to increase adult female survivorship (Fox 1993a), egg production (Credland and Wright 1989; Fox 1993a), egg size, and larval survivorship, but, contrary to the suggestion of Wasserman and Asami (1985), multiple mating had no detectable influence on offspring development time or body size.
Similar content being viewed by others
References
Begon M, Parker GA (1986) Should egg size and clutch size decrease with age? Oikos 47:293–302
Berger A (1989) Egg weight, batch size and fecundity of the spotted stalk borer, Chilo partellus in relation to weight of females and time of oviposition. Entomol Exp Appl 50:199–207
Boggs CL (1986) Reproductive strategies of female butterflies: variation in and constraints on fecundity. Ecol Entomol 11:7–15
Boggs CL (1990) A general model of the role of male-donated nutrients in female insects' reproduction. Am Nat 136:598–617
Boggs CL, Gilbert LE (1979) Male contribution to egg production in butterflies: evidence for transfer of nutrients at mating. Science 206:83–84
Boggs CL, Watt WB (1981) Population structure of pierid butterflies. IV. Genetic and physiological investment in offspring by male Colias. Oecologia 50:320–324
Boucher L, Huignard J (1987) Transfer of male secretions from the spermatophore to the female insect in Caryedon serratus (Ol.): analysis of the possible trophic role of these secretions. J Insect Physiol 33:949–957
Bowen BJ, Codd CG, Gwynne DT (1984) The katydid spermatophore (Orthoptera: Tettigoniidae): male nutritional investment and its fate in the female. Aust J Zool 32:23–31
Bownes M, Partridge L (1987) Transfer of molecules from ejaculate to females in Drosophila melanogaster and Drosophila pseudoobscura. J Insect Physiol 12:941–947
Butlin RK, Woodhatch CW, Hewitt GM (1987) Male spermatophore investment increases female fecundity in a grasshopper. Evolution 41:221–225
Carlberg U (1991) Egg-size variation in Extatosoma tiaratum (MacLeay) and its effect on survival and fitness of newly hatched nymphs (Insecta; Phasmida). Biol Zentral bl 110:163–173
Chen PS (1984) The functional morphology and biochemistry of insect male accessory glands and their secretions. Annual Review of Entomology 29:233–255
Credland PF, Wright AW (1989) Factors affecting female fecundity in the cowpea seed beetle, Callosobruchus maculatus (Coleoptera: Bruchidae). J Stored Prod Res 25:125–136
Engebretson JA, Mason WH (1980) Transfer of 65Zn at mating in Heliothis virescens. Environ Entomol 9:119–121
Engelmann F (1970) The Physiology of Insect Reproduction. Pergamon Press, Oxford
Fox CW (1993a) Multiple mating, lifetime fecundity and female mortality of the bruchid beetle, Callosobruchus maculatus (Coleoptera, Bruchidae). Funct Ecol 7:203–208
Fox CW (1993b) A quantitative genetic analysis of oviposition preference and larval performance on two hosts in the bruchid beetle, Callosobruchus maculatus. Evolution 47:166–175
Friedel T, Gillott C (1977) Contribution ofmale-produced proteins to vitellogenesis in Melanoplus sanguinipes. J Insect Physiol 23:145–151
Greenfield MD (1982) The question of paternal investment in Lepidoptera: male-contributed proteins in Plodia interpunctella. Inter J Inverte Repro 5:323–330
Gwynne DT (1984) Courtship feeding increases female reproductive success in bushcrickets. Nature 307:361–363
Gwynne DT (1988) Courtship feeding and the fitness of female katydids (Orthoptera: Tettigoniidae). Evolution 42:545–555
Gwynne DT, Bowen BJ, Codd CG (1984) The function of the katydid spermatophore and it's role in fecundity and insemination (Orthoptera: Tettigoniidae). Aust J Zool 32:15–22
Harvey GT (1977) Mean weight and rearing performance of successive egg clusters of eastern spruce budworm (Lepidoptera: Tortricidae). Can Ent 109:487–496
Harvey GT (1983) Environmental and genetic effects on mean egg weight in spruce budworm (Lepidoptera: Tortricidae). Can Ent 115:1109–1117
Honek A (1987) Regulation of body size in a heteropteran bug, Pyrrhocoris apterus. Entomol Exp Appl 44:257–262
Huignard J (1983) Transfer and fate of male secretions deposited in the spermatophore of females of Acanthoscelides obtectus Say (Coleoptera Bruchidae). J Insect Physiology 29:55–63
Jones KN, Odendaal FJ, Ehrlich PR (1986) Evidence against the spermatophore as paternal investment in the checkerspot butterflies (Euphydryas: Nymphalidae). Am Midl Nat 116:1–6
Jones RE, Hart JR, Bull GD (1982) Temperature, size and egg production in the cabbage butterfly, Pieris rapae L. Aust J Zool 30:223–232
Karlsson B, Wiklund C (1984) Egg weight variation and lack of correlation between egg weight and offspring fitness in the wall brown butterfly Lasiommata megera. Oikos 43:376–385
Karlsson B, Wiklund C (1985) Egg weight variation in relation to egg mortality and starvation endurance of newly hatched larvae in some satyrid butterflies. Ecol Entomol 10:205–211
Kasule FK (1991) Egg size increases with maternal age in the cotton stainer bugs Dysdercus fasciatus and D. cardinalis (Hemiptera: Pyrrhocoridae). Ecol Entomol 16:345–349
Lamb RJ, Smith SM (1980) Comparisons of egg size and related life-history characteristics for two predaceous tree-hole mosquitos (Toxorhynchites). Can J Zool 58:2065–2070
Leonard DE (1970) Intrinsic factors causing qualitative changes in populations of Porthetria dispar (Lepidoptera: Lymantriidae). Can Ent 102:239–249
Markow TA (1988) Drosophila males provide a material contribution to offspring sired by other males. Funct Ecol 2:77–79
Markow TA, Ankney PF (1984) Drosophila males contribute to oogenesis in a multiple mating species. Science 224:302–303
Markow TA, Ankney PF (1988) Insemination reaction in Drosophila: found in species whose males contribute material to oocytes before fertilization. Evolution 42:1097–1101
Markow TA, Gallagher PD, Krebs RA (1990) Ejaculate-derived nutritional contribution and female reproductive success in Drosophila mojavensis (Patterson and Crow). Funct Ecol 4:67–73
Marshall LD (1990) Intraspecific variation in reproductive effort by female Parapediasia teterella (Lepidoptera: Pyralidae) and its relation to body size. Can J Zool 68:44–48
Messina FJ (1991) Life-history variation in a seed beetle: adult egg-laying vs. larval competitive ability. Oecologia 85:447–455
Moore RA, Singer MC (1983) Effects of maternal age and adult diet on egg weight in the butterfly Euphydryas editha. Ecol Entomol 12:401–408
Mousseau TA, Dingle H (1991) Meternal effects in insect life histories. Annu Rev Entomol 36:511–534
Mullins DE, Keil CB (1980) Paternal investment of urates in cockroaches. Nature 283:567–569
Murphy DD, Launer AE, Ehrlich PR (1983) The role of adult feeding in egg production and population dynamics of the checkerspot butterfly Euphydryas editha. Oecologia 56:257–263
Oberhauser KS (1989) Effects of spermatophores on male and female monarch butterfly reproductive success. Behav Ecol Sociobiol 25:237–246
Parsons PA (1964) Parental age and the offspring. Q Rev Biol 39:258–275
Pitnick S, Markow TA, Riedy MF (1991) Transfer of ejaculate and incorporation of male-derived substances by females in the Nannoptera species group (Diptera: Drosophilidae). Evolution 45:774–780
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:203–208
Richards LJ, Myers JH (1980) Maternal influences on size and emergence time of the cinnabar moth. Can J Zool 58:1452–1457
Rossiter MC (1991) Maternal effects generate variation in life history: consequences of egg weight plasticity in the gypsy moth. Funct Ecol 5:386–393
Schal C, Bell WJ (1982) Ecological correlates of paternal investment of urates in a tropical cockroach. Science 218:170–173
Sibly R, Monk K (1987) A theory of grasshopper life cycles. Oikos 48:186–194
Simmons LW (1988) The contribution of multiple mating and spermatophore consumption to the lifetime reproductive success of female field crickets (Gryllus bimaculatus). Ecol Entomol 13:57–69
Sivinski J, Smittle B (1987) Male transfer of materials to mates in the Caribbean fruit fly, Anastrepha suspensa (Diptera: Tephritidae). Flor Entomol 70:233–238
Solbreck C, Olsson R, Anderson DB, Forare J (1989) Size, life history and response to food shortage in two geographical strains of the seed bug Lygaeus equestris. Oikos 55:387–396
Sota T, Mogi M (1992) Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia 90:353–358
Steinwascher K (1984) Egg size variation in Aedes aegypti: relationship to body size and other variables. Am Midl Nat 112:76–84
Svard L, Wiklund C (1988) Fecundity, egg weight and longevity in relation to multiple matings in females of the monarch butterfly. Behav Ecol Sociobiol 23:39–43
Tatar M (1986) The induction of diapause in the pipevine swallowtail, Battus philenor. Masters thesis, Dept. Zoology, University of California, Davis, CA, USA
Tauber CA, Tauber MJ, Tauber MJ (1991) Egg size and taxon: their influence on survival and development of chrysopid hatchlings after food and water deprivation. Can J Zool 69:2644–2650
Wallin H, Chiverton PA, Ekbom BS, Borg A (1992) Diet, fecundity and egg size in some polyphagous predatory carabid beetles. Entomol Exp Appl 65:129–140
Wasserman SS, Asami T (1985) The effect of maternal age upon fitness of progeny in the southern cowpea weevil, Callosobruchus maculatus. Oikos 45:191–196
Wedell N, Arak A (1989) The wartbiter spermatophore and its effect on female reproductive output (Orthoptera: Tettigoniidae, Decticus verrucivorus). Behav Ecol Sociobiol 24:117–125
Wiklund C, Karlsson B (1984) Egg size variation in satyrid butterflies: adaptive vs. historical, “Bauplan”, and mechanistic explanations. Oikos 43:391–400
Wiklund C, Persson B (1983) Fecundity, and the relation of egg weight to offspring fitness in the speckled wood butterfly Pararge aegeria, or why don't butterfly females lay more eggs? Oikos 40:53–63
Wilkinson L (1990) Systat: The system for statistics. Systat, Inc., Evanston, IL
Wilson K, Hill L (1989) Factors affecting egg maturation in the bean weevil Callosobruchus maculatus. Physiol Entomol 14:115–126
Yuma M (1984) Egg size and variability of the firefly, Lucioloa cruciata (Coleoptera, Lampyridae). Kontyu 52:615–629
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fox, C.W. The influence of maternal age and mating frequency on egg size and offspring performance in Callosobruchus maculatus (Coleoptera: Bruchidae). Oecologia 96, 139–146 (1993). https://doi.org/10.1007/BF00318042
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318042