Abstract
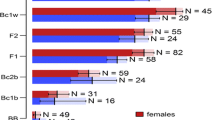
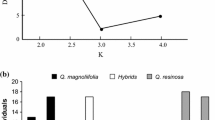
Within a population of rubber rabbitbrush, Chrysothamnus nauseosus, the subspecies C. nauseosus consimilis and C. nauseosus hololeucus, and a third unidentified group were better segregated by their insect galls, than by differences in plant morphology. This level of segregation was further increased when morphological measurements and counts of insect galls were analyzed simultaneously. We interpret this result to mean that plant morphology and insect distributions reflect two different, perhaps overlapping, portions of the host's genome. By using both sets of characters concurrently, rather than either set independently, we increased the portion of the plant's genome being sampled and increased the probability of detecting differences among host genotypes. Hence, knowledge of the distributions of insect galls may be useful for augmenting the level of separation, obtained by morphological measurements, among intrapopulational categories of plant genotypes. This application may be of greatest benefit when hybridization blurs morphological distinctions among plant taxa, when morphological traits are highly variable within genotypes, or when ephemeral morphological traits (e.g., leaves, flowers) are not available for measurements.
Similar content being viewed by others
References
Aguilar JM, Boecklen WJ (1992) Patterns of herbivory in the Quercus griseaxQuercus gambelii species complex. Oikos 64: 498–504
Akimoto S (1990) Local adaptation and host race formation of a gall-forming aphid in relation to environmental heterogeneity. Oecologia 83: 162–170
Alston RE, Turner BL (1962) New techniques in analysis of complex natural hybridization. Proc Natl Acad Sci USA 48: 130–137
Anderson LC (1966) Cytotaxonomic studies in Chrysothamnus (Asteraceae, Compositae). Am J Bot 53: 204–212
Anderson LC (1973) Unique Chrysothamnus hybridizations in Ash Meadows, Nevada, Bull Torrey Bot Club 100: 171–177
Anderson LC (1986) An overview of the genus Chrysothamnus (Asteraceae). In: McArthur, ED, Welch, BL (eds) Proceedings — Symposium on the biology of Artemisia and Chrysothamnus; (1984). GTR INT-200, US Dept Agric For Serv, pp 29–45
Askew RR (1962) The distribution of galls of Neuroterus (Hym: Cynipidae) on oak. J Anim Ecol 31: 439–455
Crego CL, Weis AE, Polans NO, Bretz CK (1990) Sympatric sibling species from three phenotypically distinct Asteromyia (Diptera: Cecidomyiidae) galls on the same host plant species. Ann Entomol Soc Am 83: 149–154
DePamphilis CW, Wyatt R (1989) Hybridization and introgression in buckeyes (Aesculus: Hippocastanaceae): a review of the evidence and a hypothesis to explain long-distance gene flow. Syst Bot 14: 593–611
Dodson GN (1991) Control of gall morphology: tephritid gallformers (Aciurina spp.) on rabbitbrush (Chrysothamnus). Ecol Entomol 16: 177–181
Dodson GN, George SB (1986) Examination of two morphs of gallforming Aciurina (Diptera: Tephritidae): ecological and genetic evidence for species. Biol J Linn Soc 29: 63–79
Fernandes GW (1992) Adaptive distribution of gall-forming insects: patterns and mechanisms. PhD thesis, Northern Arizona University, Flagstaff, Arizona
Fernandes GW, Price PW (1988) Biogeographical gradients in galling species richness: tests of hypotheses. Oecologia 76: 161–167
Floate KD, DeClerck-Floate R (1993) The role of plant development and architecture in regulating sawfly populations. In: Wagner M, Raffa KF (eds) Sawfly life history adaptations to woody plants. Academic Press, New York, pp 363–398
Floate KD, Whitham TG (1995) Insects as traits in plant systematics: their use in discriminating between hybrid cottonwoods. Can J Bot 73: 1–13
Floate KD, Whitham TG, Keim P (1994) Morphological versus genetic markers in classifying hybrid plants. Evolution 48: 929–930
Fritz RS, Price PW (1988) Genetic variation among plants and insect community structure: willow and sawflies. Ecology 69: 845–856
Fritz RS, Nichols-Orians CM, Brunsfeld SJ (1994) Interspecific hybridization of plants and resistance to herbivores: hypotheses, genetics, and variable responses in a diverse herbivore community. Oecologia 97: 106–117
Gagné RJ (1989) The plant-feeding gall midges of North America. Cornell University Press, New York
Hails RS, Crawley MJ (1991) The population dynamics of an alien insect: Andricus quercuscalicis (Hymenoptera: Cynipidae). J Anim Ecol 60: 545–562
Hanks DL, McArthur ED, Plummer AP, Guinta BC, Blauer AC (1975) Chromatographic recognition of some palatable and unpalatable subspecies of rubber rabbitbrush in and around Utah. J Range Manage 28: 144–148
Harvey SJ, Nowierski RM, Mahlberg PG, Story JM (1988) Taxonomic evaluation of leaf and latex variability of leafy spurge (Euphorbia spp.) for Montana and European accessions. Weed Sci 36: 726–733
Hegerhorst D, Weber DW, McArthur ED (1987) Resin and rubber content in Chrysothamnus. Southwest Nat 32: 475–482
Hillis DM (1987) Molecular versus morphological approaches to systematics. Annu Rev Ecol Syst 18: 23–42
Klier K, Leoschke MJ, Wendel JF (1991) Hybridization and introgression in white and yellow ladyslipper orchids (Cypripedium candidum and C. pubsecens). J Hered 82: 305–318
Mani MS (1964) The ecology of plant galls. Junk, The Hague, The Netherlands.
Matthews REF (1991) Plant virology, 3rd edn. Academic Press, New York
McArthur ED (1986) Specificity of galls on Chrysothamnus nauseosus subspecies. In: McArthur ED, Welch BL (eds) Proceedings-Symposium on the biology of Artemisia and Chrysothamnus; (1984). GTR INT-200, US Dept Agric For Serv, pp 205–210
McArthur ED, Tiernan CF, Welch BL (1979) Subspecies specificity of gall forms on Chrysothamnus nauseosus. Great Basin Nat 39: 81–87
McArthur ED, Meyer SE, Weber DJ (1987) Germination rate at low temperature: rubber rabbitbrush population differences. J Range Manage 40: 530–533
McDougall WB (1973) Seed plants of northern Arizona. The Museum of Northern Arizona, Flagstaff, Arizona
Miller JR, Strickler KL (1984) Finding and accepting host plants. In: Bell WJ, Cardé RT (eds) Chemical ecology of insects. Chapman and Hall, London, pp 127–157
Morrow PA, Whitham TG, Potts BM, Ladiges P, Ashton DH, Williams JB (1994) Gall-forming insects concentrate on hybrid phenotypes of eucalyptus. In: Price PW, Mattson WJ, Baranchikov YN (eds) The ecology and evolution of gall-forming insects. GTR NC-174, US Dept Agric For Serv, pp 121–134
Palmer MA (1952) Aphids of the Rocky Mountain region. (Thomas Say Foundation, vol. 5) The A.B. Hirschfeld Press, Denver
Patterson C, Williams DM, Humphries CJ (1993) Congruence between molecular and morphological phylogenies. Annu Rev Ecol Syst 24: 153–188
Pryor LD (1976) The biology of the eucalyptus. Camelot Press, Southampton
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43: 223–225
Schowalter TD, Haverty MI (1989) Influence of host genotype on Douglas-fir seed losses to Contarinia oregonensis (Diptera: Cecidomyiidae) and Megastigmus spermotrophus (Hymenoptera: Torymidae) in western Oregon. Environ Entomol 18: 94–97
Shorthouse JD (1982) Resource exploitation by gall wasps of the genus Diplolepis. In: Visser JH, Minks AK (eds) Proceedings of the 5th International Symposium of Insect-Plant Relationships. Wageningen. Pudoc, Wageningen, pp 193–198
Wangberg JK (1978) Biology of gall-formers of the genus Valentibulla (Diptera: Tephritidae) on rabbitbrush in Idaho. J Kansas Entomol Soc 51: 472–483
Wangberg JK (1981) Gall-forming habits of Aciurina species (Diptera: Tephritidae) on rabbitbrush (Compositae: Chrysothamnus spp.) in Idaho. J Kansas Entomol Soc 54: 711–732
Whitham TG, Morrow PA, Potts BM (1994) Plant hybrid zones as centers of biodiversity: the herbivore community of two endemic tasmanian eucalypts. Oecologia 97: 481–490
Wilkinson L (1990) SYSTAT: The system for statistics. SYSTAT, Evanston, Illinois
Zar JH (1984) Biostatistical analysis. Prentice-Hall, New Jersey
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Floate, K.D., Fernandes, G.W. & Nilsson, J.A. Distinguishing intrapopulational categories of plants by their insect faunas: galls on rabbitbrush. Oecologia 105, 221–229 (1996). https://doi.org/10.1007/BF00328550
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00328550