Summary
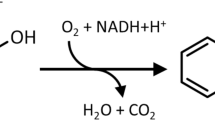
Toluate 1,2-dioxygenase is the first enzyme of a meta-cleavage pathway for the oxidative catabolism of benzoate and substituted benzoates to Krebs cycle intermediates that is specified by TOL plasmid pWW0 of Pseudomonas putida. A collection of derivatives harbouring Tn1000 insertions and defective in toluate dioxygenase have been isolated from pPL392, a pBR322-based hybrid plasmid carrying the TOL plasmid meta-cleavage pathway operon. In parallel, a series of N-methyl-N′-nitro-N-nitrosoguanidine-induced mutant plasmids defective in this enzyme activity were isolated from pNM72, a pKT231-based hybrid plasmid carrying the same operon. Pairs of mutant plasmids, consisting of one Tn1000 derivative and one nitrosoguanidine-induced derivative, were used for complementation analysis of toluate dioxygenase in Escherichia coli recA bacteria, in which the formation of 2-hydroxymuconic semialdehyde from benzoate was examined. Four cistrons for toluate 1,2-dioxygenase were thus identified. DNA fragments containing nitrosoguanidine-induced mutant cistrons plus the other meta-cleavage operon genes were cloned into pOT5, an R388-based vector, and complementation tests between different nitrosoguanidine-induced mutant cistrons were carried out in Pseudomonas putida cells, this time scoring for growth on p-toluate. This analysis also identified four cistrons. Examination of the products of these cistrons, by means of E. coli minicells containing pPL392 or its Tn1000 insertion derivatives, indicated that the first two cistrons of the operon comprise a single gene, xylX, which encodes a 57 kilodalton protein, and that the third cistron, xy/Y, encodes a 20 kilodalton protein.
Similar content being viewed by others
References
Adelberg EA, Mandel M, Chen GCC (1965) Optimal conditions for mutagenesis by N-methyl-N′-nitrosoguanidine in Escherichia coli K12. Biochem Biophys Res Commun 18:788–795
Axcell BC, Geary PJ (1975) Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J 146:173–183
Bagdasarian M, Luiz R, Rückert B, Franklin FCH, Bagdasarian MM, Frey J, Timmis KN (1981) Specific-purpose cloning vectors. II. Broad host range, high copy number RSF1010-derived vectors, and a host: vector system for gene cloning in Pseudomonas. Gene 16:237–247
Bauchop T, Eldsen SR (1960) The growth of microorganisms in relation to their energy supply. J Gen Microbiol 23:457–469
Bayley SA, Duggleby CJ, Worsey MJ, Williams PA, Hardy KG, Broda P (1977) Two modes of loss of the TOL function from Pseudomonas putida mt-2. Mol Gen Genet 154:203–204
Ensley BD, Gibson DT (1983) Naphthalene dioxygenase: Purification and properties of a terminal oxygenase component. J Bacteriol 155:505–511
Ensley BD, Gibson DT, Laborde AL (1982) Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB9816. J Bacteriol 149:948–954
Finette BA, Subramanian V, Gibson DT (1984) Isolation and characterization of Pseudomonas putida PpF1 mutants defective in the toluene dioxygenase enzyme system. J Bacteriol 160:1003–1009
Franklin FCH, Bagdasarian M, Bagdasarian MM, Timmis KN (1981) Molecular and functional analysis of the TOL plasmid pWW0 from Pseudomonas putida and cloning of genes of the entire regulated aromatic ring meta-cleavage pathway. Proc Natl Acad Sci USA 78:7458–7462
Franklin FCH, Lehrbach PR, Lurz R, Rueckert B, Bagdasarian M, Timmis KN (1983) Localization and functional analysis of transposon mutations in regulatory genes of the TOL catabolic pathway. J Bacteriol 154:676–685
Fujisawa H, Yamaguchi M, Yamaguchi T (1976) Evidence for participation of NADH-dependent reductase in the reaction of benzoate 1,2-dioxygenase. Adv Exp Med Biol 74:118–126
Geary PJ, Dickson DPE (1981) Mössbauer spectroscopy studies of the terminal dioxygenase protein of benzene dioxygenase from Pseudomonas putida. Biochem J 195:199–203
Gibson DT, Subramanian V (1984) Microbial degradation of aromatic hydrocarbons. In: Gibson DT (ed) Microbial degradation of organic compounds. Marcel Dekker, New York, pp 181–252
Gibson DT, Yeh W-K, Liu T-N, Subramanian V (1982) Toluene dioxygenase: a multicomponent enzyme system from Pseudomonas putida. In: Nozaki M, Yamamoto S (eds) Oxygenase and oxygen metabolism. Academic Press, New York, pp 51–61
Harayama S, Don RH (1985) Catabolic plasmids: their analysis and utilization in the manipulation of bacterial metabolic activities. In: Setlow JK, Hollaender A (eds) Genetic engineering: principles and methods, Vol 7. Plenum Press, New York, pp 283–307
Harayama S, Tsuda M, Iino T (1980) High frequency mobilization of the chromosome of Escherichia coli by a mutant of plasmid RP4 temperature-sensitive for maintenance. Mol Gen Genet 180:47–56
Harayama S, Engström P, Wolf-Watz H, Iino T, Hazelbauer GL (1982) Cloning of trg, a gene for sensory transducer in Escherichia coli. J Bacteriol 152:372–383
Harayama S, Lehrbach PR, Timmis KN (1984a) Transposon mutagenesis analysis of meta-cleavage pathway operon genes of the TOL plasmid of Pseudomonas putida mt-2. J Bacteriol 160:251–255
Harayama S, Oguchi T, Iino T (1984b) Does Tn10 transpose via the cointegrate molecule? Mol Gen Genet 194:444–450
Holmes DS, Quigley M (1981) A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem 114:193–197
Iida A, Harayama S, Iino T, Halzelbauer GL (1984) Molecular cloning and characterization of genes required for ribose transport and utilization in Escherichia coli K-12. J Bacteriol 158:674–682
Inouye S, Nakazawa A, Nakazawa T (1981a) Molecular cloning of TOL genes xylB and xylE in Escherichia coli. J Bacteriol 145:1137–1143
Inouye S, Nakazawa A, Nakazawa T (1981b) Molecular cloning of gene xylS of the TOL plasmid: evidence for positive regulation of the xylDEGF operon by xylS. J Bacteriol 148:413–418
Inouye S, Nakazawa A, Nakazawa T (1984) Nucleotide sequence of the promoter region of the xylDFGF operon on TOL plasmid of Pseudomonas putida. Gene 29:323–330
Kunz DA, Ribbons DW, Chapman PJ (1981) Metabolism of allylglycine and cis-crotylglycine by Pseudomonas putida (arvilla) mt-2 harboring a TOL plasmid. J Bacteriol 148:72–82
Lehrbach PR, Zeyer J, Reineke W, Knackmuss W-J, Timmis KN (1984) Enzyme recruitment in vitro: use of cloned genes to extend the range of haloaromatics degraded by Pseudomonas sp. strain B13. J Bacteriol 158:1025–1032
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, New York
Mermod N, Lehrbach PR, Reineke W, Timmis KN (1984) Transcription of the TOL plasmid toluate catabolic pathway operon of Pseudomonas putida is determined by a pair of co-ordinately and positively regulated overlapping promoters. EMBO J 3:2461–2466
Mermod N, Lehrbach PR, Don RH, Timmis KN (1986) Gene cloning and manipulation in Pseudomonas. In: Sokatch JR (ed) The bacteria, Vol 10, Academic Press, New York, in press
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Nakazawa T, Inouye S, Nakazawa A (1980) Physical and functional mapping of RP4-TOL plasmid recombinants: analysis of insertion and deletion mutants. J Bacteriol 144:222–231
Reineke W, Knackmuss H-J (1978) Chemical structure and biodegradability of hologenated aromatic compounds. Substituent effects on 1,2-dioxygenation of benzoic acid. Biochim Biophys Acta 542:412–423
Reineke W, Knackmuss H-J (1980) Hybrid pathway for chlorobenzoate metabolism in Pseudomonas sp. B13 derivatives. J Bacteriol 142:467–473
Sauber K, Fröhner C, Rosenberg G, Eberspächer J, Lingens F (1977) Purification and properties of pyrazon dioxygenase from pyrazon-degrading bacteria. Eur J Biochem 74:89–97
Subramanian V, Liu T-N, Yeh W-K, Gibson DT (1979) Toluene dioxygenase: purification of an iron-sulfur protein by affinity chromatography. Biochem Biophys Res Commun 91:1131–1139
Subramanian V, Liu TN, Yeh W-K, Narro M, Gibson DT (1981) Purification and properties of NADH-ferredoxinTOL reductase. A component of toluene dioxygenase from Pseudomonas putida. J Biol Chem 256:2723–2730
Williams PA, Murray K (1974) Metabolism of benzoate and methylbenzoate by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol 120:416–423
Worsey MJ, Franklin FCH, Williams PA (1978) Regulation of the degradative pathway enzymes coded for by the TOL plasmid (pWW0) from Pseudomonas putida mt-2. J Bacteriol 134:757–767
Yamaguchi M, Fujisawa H (1978) Characterization of NADH-cytochrome c reductase, a component of benzoate 1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol Chem 253:8848–8853
Yamaguchi M, Fujisawa H (1980) Purification and characterization of an oxygenase component in benzoate 1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol Chem 255:5058–5063
Yamaguchi M, Fujisawa H (1981) Reconstitution of iron-sulphur cluster of NADH-cytochrome c reductase, a component of benzoate 1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol chem 256:6783–6787
Yamaguchi M, Fujisawa H (1982) Subunit structure of oxygenase component in benzoate-1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol Chem 257:12497–12502
Yeh W-K, Gibson DT, Liu T-N (1977) Toluene dioxygenase: a multicomponent enzyme system. Biochem Biophys Res Commun 78:401–410
Author information
Authors and Affiliations
Additional information
Communicated by K. Isono
Rights and permissions
About this article
Cite this article
Harayama, S., Rekik, M. & Timmis, K.N. Genetic analysis of a relaxed substrate specificity aromatic ring dioxygenase, toluate 1,2-dioxygenase, encoded by TOL plasmid pWW0 of Pseudomonas putida . Mol Gen Genet 202, 226–234 (1986). https://doi.org/10.1007/BF00331641
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00331641