Summary
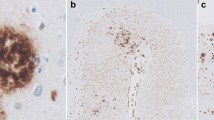
To investigate the neuropathological differences between normal aging and senile dementia of the Alzheimer type (SDAT) in very old people and to see how they compare with a younger population of demented elderly people, we performed an immunohistochemical quantitative analysis of the topography of senile plaques and neurofibrillary tangles in a series of 31 elderly patients aged from 96 to 102 years. According to the medical records, two groups were considered: 7 patients presenting with clinically documented SDAT and 24 patients with no or very mild cognitive impairment. The densities of senile plaques were comparable in both groups. Extensive neurofibrillary tangle formation was restricted to the CA1 hippocampal field of demented subjects, whereas the superior frontal cortex showed rare neurofibrillary tangles, independently of the clinical diagnosis. These results indicate an absence of direct correlation between the number of senile plaques and the clinical manifestation of SDAT. Furthermore, they suggest that the dementing process may involve different cortical structures in nonagenarians and centenarians than in younger demented individuals where a widespread cortical involvement is generally observed. Thus, the neurofibrillary tangle density in the CA1 field may be critical for the neuropathological diagnosis of SDAT in this particular group of very old patients.
Similar content being viewed by others
References
Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cerebral Cortex 1:103–116
Arriagada PV, Growdon JH, Hedley-Whyte T, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 42:631–639
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology 42:1681–1688
Blessed G, Tomlinson BE, Roth M (1968) The association between quantitative measures of dementia and of senile change in the cerebral grey matter of the elderly subjects. Br J Psychiatry 114:797–811
Bouras C, Giannakopoulos P, Hof PR, Robakis NK, Surini M, Michel JP (1992) Distribution of neurofibrillary rangles and amyloid deposits in the hippocampus and the temporal neocortex: a study of a one year autopsy population from a geriatric hospital. Soc Neurosci Abstr 18:565
Brun A, Gustafson L, Samuelsson SM, Ericsson C (1992) Neuropathology of late life. Dementia 3:125–130
Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M and Wolfson L (1988) Clinico-pathological studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology 38:1682–1687
Dayan AD (1970) Quantitative histological studies on the aged human brain. I. Senile plaques and neurofibrillary tangles in normal patients. Acta Neuropathol (Berl) 16:85–94
Delacourte A, Flament S, Dibe EM, Hublau P, Sablonnière B, Hémon B, Scherrer V, Défossez A (1990) Pathological proteins Tau 64 and 69 are specifically expressed in the somatodendritic domain of the degenerating cortical neurons during Alzheimer's disease. Demonstration with a panel of antibodies against Tau proteins. Acta Neuropathol 80:111–117
Evans DA, Scherr PA, Cook NA, Albert MS, Funkenstein HH, Beckett LA, Hebert LE, Wetle TT, Branch LG, Chown MJ, Hennekens CH, Taylor JO (1992) The impact of Alzheimer's disease in the United States population. In: Willis DP, Manton KG (eds) The oldest-old. Suzman RM. Oxford University Press, New York, pp 283–299
Flament S, Delacourte A, Delaère P, Duyckaerts C, Hauw JJ (1990) Correlations between microscopical changes and tau 64 and 69 biochemical detection in senile dementia of the Alzheimer's type. Tau 64 and 69 are reliable markers of the neurofibrillary degeneration. Acta Neuropathol 80:212–215
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Guntern R, Vallet PG, Bouras C, Constantinidis J (1989) An improved immunohistostaining procedure for peptides in human brain. Experientia 45:159–161
Hauw JJ, Vignolo P, Duyckaerts C, Beck H, Forette F, Henry JF, Laurent M, Piette F, Sachet A, Berthaux P (1986) Etude neuropathologique de 12 centenaires: la fréquence de la démence sénile de type Alzheimer n'est pas particulièrement élevée dans ce groupe de personnes très âgées. Rev. Neurol (Paris) 142:107–115
Hof PR, Morrison JH (1990) Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease. II. Primary and secondary visual cortex. J Comp Neurol 301:55–64
Hof PR, Cox K, Morrison JH (1990) Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease. I. Superior frontal and inferior temporal cortex. J Comp Neurol 301:44–54
Hof PR, Bierer LM, Perl DP, Delacourte A, Buée L, Bouras C, Morrison JH (1992) Evidence for early vulnerability of the medial and inferior aspects of the temporal lobe in an 82-year-old patient with preclinical signs of dementia. Regional and laminar distribution on neurofibrillary tangles and senile plaques. Arch Neurol 49:946–953
Howell TH, Piggot AP (1951) Morbid anatomy of old age. Geriatrics 6:85–95
Hubbard BM, Anderson JM (1981) A quantitative study of cerebral atrophy in old age and senile dementia. J Neurol Sci 50:135–145
Hubbard BM, Fenton GW, Anderson JM (1990) A quantitative histological study of early clinical and preclinical Alzheimer's disease. Neuropathol Appl Neurobiol 16:111–121
Hyman BT, Van Hoesen GW, Damasio AR (1990) Memoryrelated systems in Alzheimer's disease: an anatomic study. Neurology 40:1721–1730
Karasawa A (1985) Changes in intellectual ability in normal human aging. Shinkei Kenkuo No Shinpo 29:536–546
Katzman R, Terry RD (1983) Normal aging of the nervous system. In: Katzman R, Terry RD (eds) The neurology of aging. Davis, Philadelphia, pp 15–50
Khachaturian ZS (1985) Diagnosis of Alzheimer's disease. Arch Neurol 42:1097–1105
Kim KS, Miller DL, Sapienza VG, Chen CJ, Vai C, Grundke-Iqbal I, Curry JR, Wisniewski HM (1988) Production and characterization of monoclonal antibodies reactive to synthetic cerebrovascular amyloid peptide. Neurosci Res Commun 2:121–130
Lewis DA, Campbell MJ, Terry RD, Morrison JH (1987) Laminar and regional distribution of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual cortex and auditory cortices. J Neurosci 7:1799–1808
Matsuyama H, Nakamura S (1978) Senile changes in the brain in the Japanese: incidence of Alzheimer's neurofibrillar change and senile plaques. In: Katzman R, Terry RD, Bick KL (eds) Alzheimer's disease: senile dementia and related disorders. Raven Press, New York, pp 287–297
Mizutani T, Shimada H (1990) Neuropathological aspects of centenarian brains: study of 24 autopsy cases. In: Hasegawa K, Homma A (eds) Psychogeriatrics-biomedical and social advances. Excerpta Medica, Tokyo, pp 128–133
Mizutani T, Shimada H (1992) Neuropathological background of twenty-senven centenarian brains. J Neurol Sci 108:168–177
Morris JC, McKell JDW, Storandt M, Rubin EH, Price JL, Grant EA, Ball MJ, Berg L (1991) Very mild Alzheimer's disease: informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology 41:469–478
Pearson RCA, Esiri MM, Hiorns RW, Wilcock GK, Powell TPS (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer's disease. Proc Natl Acad Sci USA 82:4531–4534
Peress NS, Kane WC, Aronson SM (1973) Central nervous system findings in a tenth decade autopsy population. Prog Brain Res 40:470–483
Price JL, Davis PB, Morris JC, White DL (1991) The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 12:295–312
Rogers J, Morrison JH (1985) Quantitative morphology and regional and laminar distribution of senile plaques in Alzheimer's disease. J Neurosci 5:2801–2808
Tomlinson BE, Blessed G, Roth M (1968) Observations on the brains of non-demented old people. J Neurol Sci 7:331–356
Ulrich J (1985) Alzheimer changes in non-demented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol 17:273–277
Vallet PG, Guntern R, Hof PR, Golaz J, Delacourte A, Robakis NK, Bouras C (1992) A comparative study of histological and immunohistochemical methods for neurofibrillary tangles and senile plaques in Alzheimer's disease. Acta Neuropathol 83:170–178
Vermersch P, Frigard B, David JP, Fallet-Bianco C, Delacourte A (1992) Presence of abnormally phosphorylated Tau proteins in the entorhinal cortex of aged non-demented subjects. Neurosci Lett 144:143–146
Author information
Authors and Affiliations
Additional information
Supported by grants from the American Health Assistance Foundation and the Brookdale Foundation (to P.R.H.)
Rights and permissions
About this article
Cite this article
Giannakopoulos, P., Hof, P.R., Surini, M. et al. Quantitative immunohistochemical analysis of the distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of nonagenarians and centenarians. Acta Neuropathol 85, 602–610 (1993). https://doi.org/10.1007/BF00334669
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00334669