Summary
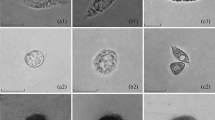
The fine structure of oyster leucocytes resembles to a great extent, that of typical eucaryotic cells. Organelles which have been described for the first time in this report are light granules, dense granules, protocentriole and X structure. Light microscopy reveals two morphological types of oyster leucocytes: agranular and granular. Based upon nuclear morphology and cytoplasmic compositions revealed in electron microscopy, at least three types of agranular and one type of granular cells are recognized.
In the Giemsa-stained preparations, granular leucocytes exhibit three distinct types of cytoplasmic granules: refractile, dark blue, and pink, which presumably correspond to light granules Type A, B, and C seen in the electron micrographs. A granular leucocyte may contain one or more types of granules. Cytochemical investigations show that oyster leucocytes contain at least three hydrolytic enzymes: non-specific esterases, acid, and alkaline phosphatase. The latter two enzymes constitute 63% of the enzyme activity detected. These intracellular enzymes may be associated with the light granules and/or lysosome-like bodies.
It is also demonstrated that the granular leucocyte population is significantly higher (P<0.001) in the oysters experimentally infected with Bacillus mycoides (72.19±4.71%) as contrasted with that of the controls (37.18±4.48%).
Leucocytes in progressive stages of degeneration are also described.
Similar content being viewed by others
References
Archer, G. T., Hirsch, J. G.: Isolation of granules from eosinophil and study of their enzyme content. J. exp. Med. 118, 277–286 (1963a).
—: Motion picture studies on degranulation of horse eosinophils during phagocytosis. J. exp. Med. 118, 287–293 (1963b).
Bainton, D. F., Farquhar, M. G.: Origin of granules in polymorphonuclear leucocytes. J. Cell Biol. 28, 277–301 (1966).
—: Segregation and packaging of granule enzymes in eosinophilic leukocytes. J. Cell Biol. 45, 54–73 (1970).
Balazs, A.: Granule formation in rat myeloid cells. An electron microscopic study. Z. Zellforsch. 99, 286–301 (1969).
Burstone, M. S.: The cytochemical localization of esterase. J. nat. Cancer Inst. 18, 167–172 (1957).
—: Histochemical comparison of naphthol AS-phosphates for the demonstration of phosphatases. J. Nat. Cancer Inst. 20, 601–616 (1958).
Caro, L. G., Palade, G. E.: Protein synthesis, storage and discharge in the pancreatic exocrine cell. J. Cell Biol. 20, 473–495 (1964).
Cheng, T. C., Rifkin, E.: Cellular reactions in marine molluscs in response to helminth parasitism. In: Symposium on Diseases of Fishes and Shellfishes, S. F. Snieszko, Ed. Amer. Fisheries Soc. Special Publ. No. 5, 443–496 (1970).
Chestnut, A. F.: Studies on the digestive processes of Ostrea mrginica. Doctoral Thesis, Rutgers, The State University, New Brunswick, N. J. pp. 88 (1949).
Cohn, Z. A., Hirsch, J. G.: The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J. exp. Med. 112, 983–1104 (1960).
Duve, C. de: The lysosome content. Ciba Foundation Symposium on Lysosomes (A. V. S. deReuck and M. P. Cameron, eds.), pp. 1–28. Boston: Little, Brown & Co. 1963.
Eble, A. F.: Some observations on the seasonal distribution of selected enzymes in the American oyster as revealed by enzyme histochemistry. Pros. Nat. Shellfish. Ass. 56, 37–42 (1966).
—, Tripp, M. R.: Enzyme histochemistry of phagosomes in oyster leucocytes. Bull. N. J. Acad. Sci. B 13, 93 (1968).
Fedorko, M. E.: Formation of cytoplasmic granules in human eosinophilic myelocytes: An electron microscope autoradiographic study. Blood 31, 188–194 (1968).
—, Hirsch, J. G.: Cytoplasmic granule formation in myelocytes. An electron microscopc radioautographic study on the mechanism of formation of cytoplasmic granules in rabbit heterophilic myelocytes. J. Cell Biol. 29, 307–316 (1966).
Feng, S. Y.: Pinocytosis of proteins by oyster leucocytes. Biol. Bull. 129, 95–105 (1965).
—: Experimental bacterial infections in the oyster Crassostrea virginica. J. Invertebrate Path. 8, 505–511 (1966).
—: Responses of molluscs to foreign bodies, with special reference to the oyster. Fed. Proc. 26, 1685–1692 (1967).
George, W. C.: The digestion and absorption of fat in lamellibranchs. Biol. Bull. 102, 118–127 (1952).
Hearing, V., Vernick, S. H.: Fine structure of the blood cells of the lobster, Homarus americanus. Chesapeake Sci. 8, 170–186 (1967).
Hirsch, J. C.: Cinematographic observations on granule lysis in polymorphonuclear leucocytes during phagocytosis. J. exp. Med. 116, 827–834 (1962).
—, Cohn, Z. A.: Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J. exp. Med. 112, 1005–1014 (1960).
Hudson, G.: Eosinophil granules and phosphotungstic acid: an electron microcope study of guinea-pig bone marrow. Exp. Cell Res. 41, 265–273 (1966).
Hunter, R. L., Burstone, M. S.: The zymogram as a tool for the characterization of enzyme substrate specificity. J. Histochem. Cytochem. 8, 58–61 (1960).
Ichikawa, Y.: Electron microscopic studies on the developing processes of granules of neutrophil leucocyte in normal and leukemic mice. Acta haemat. jap. 21, 885–895 (1958).
Lockwood, W. R., Allison, F.: Electron micrographic studies of phagocytic cells. I. Morphological changes of the cytoplasm and granules of rabbit granulocytes associated with ingestion of rough pneumococcus. Brit. J. exp. Path. 44, 593–600 (1963).
Mackin, J. G.: Oyster disease caused by Dermocystidium and other microorganisms in Louisiana. Publ. Inst. Mar. Sci. Univ. Texas, 7, 132–229 (1962).
Movat, H. Z., Weiser, W. J., Glynn, M. F., Mustard, J. F.: Platelet phagocytosis and aggregation. J. Cell Biol. 27, 531–543 (1965).
Nelson, T. C.: On the digestion of animal forms by the oysters. Proc. Soc. exp. Biol. 30, 1287–1290 (1933).
Novikoff, A. B., Essner, E., Quintana, N.: The golgi apparatus and lysosomes. Fed. Proc. 23, 1010–1022 (1964).
Perkins, F. O.: Formation of centriole and centriole-like structures during meiosis and mitosis in Labyrinthula sp. (Rhizopodea, Labyrinthulida) An electron-microscope study. J. Cell Sci. 6, 629–653 (1970).
Philpott, D. E., Chaet, A. B., Burnett, A. L.: A study of the secretory granules of the basal disk of Hydra. J. Ultrastruct. Res. 14, 74–84 (1966).
Prytherch, H. F.: The life cycle and morphology of Nematopsis ostrearum sp. nov., a gregarine parasite of the mud crab and oyster. J. Morph. 66, 39–65 (1940).
Rifkin, E., Cheng, T. C.: The origin, structure, and histochemical characterization of encapsulating cysts in the oyster Crassostrea virginica parasitized by the cestode Tylocephalum sp. J. Invertebrate Path. 10, 54–64 (1968).
Spicer, D. D., Horn, R. G., Wetzel, B. K.: Ultrastructural and cytochemical characteristics of leucocytes in various stages of development. Biochem. Pharmacol., Suppl., 143–157 (1968).
Stauber, L. A.: The fate of india ink injected intracardially into the oyster, Ostrea virginica Gmelin. Biol. Bull. 98, 227–241 (1950).
Sutton, J. S.: Weiss, L.: Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J. Cell Biol. 28, 303–332 (1966).
Takatsuki, S.: On the nature and functions of the amoebocytes of Ostrea edulis. Quart. J. micr. Sci. 76, 379–431 (1934).
Tripp, M. R.: Disposal by the oyster of intracardially injected red blood cells of vertebrates. Proc. Nat. Shellfish. Ass. 48, 143–147 (1958).
—: Mechanisms of removal of injected microorganisms from the American oyster, Crassostrea virginica (Gmelin). Biol. Bull. 199, 273–282 (1960).
—, Bisignani, L. A., Kenny, M. T.: Oyster amoebocytes in vitro. J. Invertebrate Path. 8, 137–140 (1966).
Winborn, W. B.: Dow epoxy resin with triallyl cyanurate, and similarly modified araldite and maraglas mixtures as embedding media for electron microscopy. Stain Technol. 40, 227–231 (1965).
—, Bockman, D. E.: Origin of lysosomes in parietal cells. Lab. Invest. 19, 256–264 (1968).
Yonge, C. M.: Structure and phyisology of the organs of feeding and digestion in Ostrea edulis. J. marine biol. Ass. 14, 295–386 (1926).
Zucker-Franklin, D., Hirsch, J. G.: Electron microscopic studies on the degranulation of rabbit peritoneal leucocytes during phagocytosis. J. exp. Med. 120, 569–575 (1964).
Author information
Authors and Affiliations
Additional information
Contribution No. 71 from Marine Research Laboratory, University of Connecticut.
The initial phase of this investigation was carried out at the Department of Zoology, Rutgers, The State University, New Brunswick, New Jersey, and supported by Public Health Service Research Grant AI-00781 from the National Institute of Allergy and Infectious Diseases of the National Institute of Health, awarded to Dr. L. A. Stauber. Supported by a grant from the University of Connecticut Research Foundation and Faculty Summer Fellowship to S. Y. Feng.
Rights and permissions
About this article
Cite this article
Feng, S.Y., Feng, J.S., Burke, C.N. et al. Light and electron microscopy of the leucocytes of Crassostrea virginica (Mollusca: Pelecypoda). Z. Zellforsch. 120, 222–245 (1971). https://doi.org/10.1007/BF00335537
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00335537