Abstract
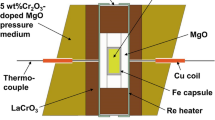
The univariant high-pressure reaction of aluminous enstatite and spinel to pyrope and forsterite in the MgO-Al2O3-SiO2 system has been determined in the temperature range 900 °–1100 °C by hydrothermal reversals in the piston-cylinder apparatus using the low-friction NaCl pressure medium. A mixture of synthetic minerals, including an enstatite with 6 wt% Al2O3, with product and reactant assemblages in nearly equal amounts, was the starting material. The equilibrium pressure of 19.3±0.3 kbar at 1000 ° C and average dP/dT slope of 8.0 bars/ ° C confirm the strong curvature of the equilibrium below 1200 ° C deduced by Obata (1976) from a theoretical study of experimental Al2O3 isopleths of enstatite in the garnet field. His prediction of an absolute minimum pressure near 18 kbar of the garnet peridotite assemblage in the ternary system is undoubtedly correct.
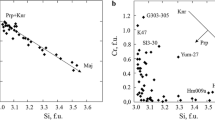
Three reversed determinations of the equilibrium Al2O3 content of enstatite in the presence of spinel +forsterite were made at points adjacent to the univariant curve. The points are 5.5 wt% Al2O3 at 950 ° C and 20 kbar, 6.2 wt% at 1000 ° C and 20 kbar and 7.2 wt% at 1080 ° C and 20 kbar. These values are somewhat higher than given by the MacGregor (1974) isopleth set and quite close to those predicted by Fujii (1976) from experimental synthesis data at higher temperatures, using the Wood and Banno (1973) model of ideal solution of the Mg2Si2O6 and MgAl2SiO6 components in enstatite to reduce the data.
All of the available spinel-field isopleth data can be systematized with the use of the ideal solution model. A value of ΔH 0 of 9000 cal fits the reduced data well, and is in agreement with the calorimetrically determined value of 8500±1900 calories. An accurate calculation of the dP/dT slope of the univariant equilibrium at 1000 ° C based on calorimetry gives 7±2bars/ °C, also in good agreement with experiment. Thus, all of the available experimental and calorimetric data are consistent with the ideal-solution aluminous enstatite model.
The dP/dT slopes of the spinel-field isopleths are too large to permit their use as an accurate geobarometric scale. They do have considerable potential as a thermometric indicator for certain natural peridotites, however. The southwestern Oregon overthrust peridotite masses of Cretaceous age have enstatite of 5.6 wt% Al2O3 with spinel of nearly 80 mole% MgAl2O4. The present reduced isopleth data directly give 930 ° C for the equilibration, assuming 12 kbar pressure. A first order correction based on ideal solution departures from the ternary system, as suggested by Stroh (1976) gives 1000 ° C. Thus, the high temperatures deduced by Medaris (1972) are confirmed. The pressure cannot be deduced independently from the pyroxene Al2O3 contents.
Similar content being viewed by others
References
Ahrens, T.J.: The mineralogic distribution of iron in the upper mantle. Phys. Earth Planet. Inter. 5, 267–281 (1972)
Anastasiou, P., Seifert, F.: Solid solubility of Al2O3 in enstatite at high temperatures and 1–5 kb water pressure. Contrib. Mineral. Petrol. 34, 272–287 (1972)
Boyd, F.R.: A pyroxene geotherm. Geochim. Cosmochim. Acta 37, 2533–2546 (1973)
Boyd, F.R., England, J.L.: The system enstatite-pyrope. Carnegie Inst. Wash. Year Book 63, 157–161 (1964)
Boyd, F.R., England, J.L.: The rhombic enstatite-clinoenstatite inversion. Carnegie Inst. Wash. Year Book 64, 117–120 (1965)
Burnham, C.W.: Lattice constant refinement. Carnegie Inst. Wash. Year Book 61, 132–135 (1962)
Charlu, T.V., Newton, R.C., Kleppa, O.J.: Enthalpies of solution at 970 K, of compounds in the system MgO-Al2O3-SiO2 by high temperature solution calorimetry. Geochim. Cosmochim. Acta 39, 1487–1497 (1975)
Chatterjee, N.D., Schreyer, W.: The reaction enstatite+sillimanite = sapphirine+quartz in the system MgO-Al2O3-SiO2. Contrib. Mineral. Petrol. 36, 49–62 (1972)
Davis, B.T.C., Boyd, F.R.: The join Mg2Si2O6-CaMgSi2O6 at 30 kb and its application to pyroxenes from kimberlites. J. Geophys. Res. 7, 3567–3576 (1966)
Dixon, J.R., Presnall, D.C.: Geothermometry and geobarometry of synthetic spinel lherzolite in the system CaO-MgO-Al2O3- SiO2. Extended Abstr., second Kimberlite Conference, Santa Fe, N. Mex., Oct. 3–7, 1977.
Fujii, T.: Solubility of Al2O3 in enstatite coexisting with forsterite and spinel. Carnegie Inst. Wash. Year Book 75, 566–571 (1976)
Ganguly, J., Ghose, S.: Order-disorder in aluminous orthopyroxene and its petrologic implications. Geol. Soc. Am. Ann. Mtg. Progr. with Abstr., 984 (1977)
Goldsmith, J.R., Newton, R.C.: An experimental determination of the alkali feldspar solvus. In: The feldspars (W.S. MacKenzie and J. Zussman, eds.,), pp. 337–359. Manchester: University Press 1974
Hays, J.F., Bell, P.M.: Albite-jadeite-quartz equilibrium: a hydrostatic determination. Carnegie Inst. Wash. Year Book 72, 706–708 (1973)
Johannes, W.: A simplified piston-cylinder apparatus of high precision. Neues Jahrb. Mineral. Monatsh. 7/8, 337–351 (1973)
Johannes, W., Bell, P.M., Mao, H.K., Boettcher, A.L., Chipman, D.W., Hays, J.F., Newton, R.C., Seifert, F.: An interlaboratory comparison of piston-cylinder pressure calibration using the albite breakdown reaction. Contrib. Mineral. Petrol. 32, 24–38 (1971)
Kushiro, I., Yoder, H.S.: Anorthite-forsterite and anorthite-enstatite reactions and their bearing on the basalt-eclogite transformation. J. Petrol. 7, 337–362 (1966)
MacGregor, I.D.: The reaction 4 enstatite+spinel ⇄ forsterite+pyrope. Carnegie Inst. Wash. Year Book 63, 157 (1964)
MacGregor, I.D.: Stability fields of spinel and garnet peridotites in the synthetic system MgO-CaO-Al2O3-SiO2. Carnegie Inst. Wash. Year Book 64, 126–134 (1965)
MacGregor, I.D.: The system MgO-Al2O3-SiO2: solubility of Al2O3 in enstatite for spinel and garnet peridotite compositions. Am. Mineralogist 59, 110–119 (1974)
MacGregor, I.D., Basu, A.: Thermal structure of the lithosphere: a petrologic model. Science 185, 1007–1011 (1974)
Medaris, L.G.: High-pressure peridotites in southwestern Oregon. Geol. Soc. Am. Bull. 83, 41–58 (1972)
Mercier, J.-C., Carter, N.L.: Pyroxene geotherms. J. Geophys. Res. 80, 3349–3362 (1975)
Mirwald, P.W., Getting, I.C., Kennedy, G.C.: Low-friction cell for piston-cylinder high-pressure apparatus. J. Geophys. Res. 80, 1519–1525 (1975)
Navrotsky, A., Kleppa, O.J.: The thermodynamics of formation of simple spinels. J. Inorg. Nucl. Chem. 30, 479–498 (1968)
Newton, R.C.: Kyanite-sillimanite equilibrium at 750 ° C. Science 151, 1222–1225 (1966)
Newton, R.C., Smith, J.V.: Investigations concerning the breakdown of albite at depth in the earth. J. Geol. 75, 268–286 (1967)
Newton, R.C., Thompson, A.B., Krupka, K.M.: Heat capacity of synthetic Mg3Al2Si3O12 from 350 to 1000 K and the entropy of pyrope. EOS 58, 523 (1977)
Obata, M.: The solubility of Al2O3 in orthopyroxenes in spinel and plagioclase peridotites and spinel pyroxenite. Am. Mineralogist 61, 804–816 (1976)
O'Hara, M.J.: Mineral parageneses in ultrabasic rocks, In: Ultramafic and related rocks (P.J. Wyllie, ed.), pp. 393–403. J. Wiley 1967
O'Hara, M.J., Mercy, E.L.P.: Petrology and petrogenesis of some garnetiferous peridotites. Trans. Roy. Soc. Edinburgh 65, 251–314 (1963)
O'Hara, M.J., Richardson, S.W., Wilson, G.: Garnet peridotite stability and occurrence in crust and mantle. Contrib. Mineral. Petrol. 32, 48–67 (1971)
Robie, R.A., Waldbaum, D.R.: Thermodynamic properties of minerals and related substances at 298.15 K (25.0 ° C) and one atmosphere (1.013 bars) pressure and at higher temperatures. U.S. Geol. Surv. Bull. 1259, 256 (1968)
Saxena, S., Ghose, S.: Mg2+-Fe2+ order-disorder and the thermodynamics of the orthopyroxene crystalline solution. Am. Mineralogist 56, 532–559 (1971)
Skinner, B.J.: Thermal expansion, In: Handbook of physical constants (S.P. Clark, ed.), Vol. 97, pp. 75–96. Geol. Soc. Am. Mem. 1966
Skinner, B.J., Boyd, F.R.: Aluminous enstatites. Carnegie Inst. Wash. Year Book 63, 163–165 (1964)
Stephenson, D.A., Sclar, C.B., Smith, J.V.: Unit cell volumes of synthetic orthoenstatite and low clinoenstatite. Mineral. Mag. 35, 838–846 (1966)
Stroh, J.M.: Solubility of alumina in orthopyroxene plus spinel as a geobarometer in complex systems: applications to spinel-bearing alpine-type peridotites. Contrib. Mineral. Petrol. 54, 173–188 (1976)
Wood, B.J.: The application of thermodynamics to some subsolidus equilibria involving solid solutions. Fortschr. Mineral. 52, 21–45 (1975)
Wood, B.J., Banno, S.: Garnet-orthopyroxene and orthopyroxene-clinopyroxene relationships in simple and complex systems Contrib. Mineral. Petrol. 42 109–124 (1973)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Danckwerth, P.A., Newton, R.C. Experimental determination of the spinel peridotite to garnet peridotite reaction in the system MgO-Al2O3-SiO2 in the range 900 °–1100 °C and Al2O3 isopleths of enstatite in the spinel field. Contr. Mineral. and Petrol. 66, 189–201 (1978). https://doi.org/10.1007/BF00372158
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00372158