Abstract
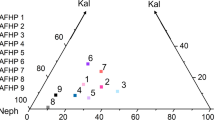
An experimental study has been carried out to determine the effect of solution composition on the partitioning behaviour of tungsten in granitic melt-vapour systems at 800° C and 1 kbar. With chloride and phosphate solutions, tungsten partitions strongly into the aqueous phase, whereas with fluoride, carbonate and borate solutions, and water alone, tungsten partitions in favour of the melt. With chloride solutions, the fluid/melt partition coefficients (K D) for W show a marked positive correlation with chloride concentration, and suggest that at low chloride concentrations W-Cl complexes with low Cl∶W ratios (such as associated equivalents of (WO3)2C1−) may be present. In contrast, at higher chloride concentrations complexes with high Cl∶W ratios (such as WOCl4, WCl6 and associated ionic equivalents) may predominate. With phosphate solutions, K D shows little variation with phosphate concentration, and phosphorus heteropolytungstates (such as H3[PW12O40]) may be present. There is no evidence to suggest that fluoride, carbonate or borate complexes of tungsten are important under the experimental conditions: the data for these compositions can be interpreted assuming that isopolytungstates (such as H6[H2W12O40]) are present. Within high temperature hydrothermal solutions tungsten may be transported principally as isopolytungstates and heteropolytungstates in addition to chloride complexes, and this may, in part, account for the common association of apatite and arsenopyrite with scheelite and wolframite in tungsten deposits.
Similar content being viewed by others
References
Chang LLY, Sachdev S (1975) Alkali tungstates: stability relations in the systems A2O · WO3 — WO3. J Am Ceram Soc 58:267–270
Charlot G, Collumeau A, Marchon MJC (1971) Selected constants. Oxidation-reduction potentials of inorganic substances in aqueous solution. Butterworth, London
Conroy LE (1977) The preparation and characterisation of a sodium tungsten bronze. J Chem Educ 54:45–49
Dickens PG, Whittingham MS (1968) The tungsten bronzes and related compounds. Q Rev Chem Soc Lond 22:30–44
Fergusson JE (1967) Halide chemistry of Cr, Mo, W. Halogen Chemistry 3:227–302
Flynn RT, Burnham CW (1978) An experimental determination of rare earth partition coefficients between a chloride containing vapour phase and silicate melts. Geochim Cosmochim Acta 42:685–701
Foster RP (1977) Solubility of scheelite in hydrothermal chloride solutions. Chem Geol 20:27–43
Foster RP, Mann AG, Armin T, Burmeister BB (1978) Richardson's Kop wolframite deposit, Rhodesia: a geochemical model for the hydrothermal behaviour of tungsten. In: WJ Verwoed (ed) Mineralisation in metamorphic terranes. Geol Soc S Afr Spec Publ 4:107–128
Freer R (1981) Diffusion in silicate minerals and glasses: a data digest and guide to the literature. Contrib Mineral Petrol 76:440–454
Gmelin (1979) Gmelin Handbuch der Anorganischen Chemie, W. Springer, Berlin Heidelberg New York
Gundlach H, Thormann W (1960) Versuch einer Deutung der Entstehung von Wolframund Zinnlagerstätten. Z Dtsch Geol Ges 112:1–35
Hamilton DL, Henderson CMB (1968) The preparation of silicate compositions by a gelling method. Mineral Mag 36:832–838
Higgins NC (1980) Fluid inclusion evidence for the transport of tungsten by carbonate complexes in hydrothermal solutions. Can J Earth Sci 17:823–830
Hofmann AW (1980) Diffusion in natural silicate melts: a critical review. In: Hargraves RB (ed) Physics of magmatic processes, Princeton University Press:385–417
Holland HD (1972) Granites, solutions and base metal deposits. Econ Geol 67:281–301
Ivanova GF (1966) Thermodynamic evaluation of the possibility of tungsten transport as halogen compounds. Geochem Int 10:964–973
Keggin JF (1934) The structure and formula of 12-phosphotungstic acid. Proc Roy Soc Lond A 144:75–100
Kepert DL (1962) Isopolytungstates. Prog Inorgan Chem 4:199–274
Kepert DL (1973) Isopolyanions and heteropolyanions. In: Bailar JC, Emeleus HJ, Nyholm S, Trotman-Dickenson AF (eds) Comprehensive inorganic chemistry, 4:607–672, Pergamon Press, Oxford
Killinc IA, Burnham CW (1972) Partitioning of chloride between a silicate melt and coexisting aqueous phase from 2 to 8 kilobars. Econ Geol 67:231–235
Manning DAC (1981 a) The use of radioactive tracers in determining the behaviour of tin in silicate-vapour systems. NERC rept Progress in Experimental Petrology 5:15–16
Manning DAC (1981 b) The effect of fluorine on liquidas phase relationships in the system Qz-Ab-Or with excess water at 1 kb. Contrib Mineral Petrol 76:206–215
Manning DAC (1984) Volatile control of tungsten partitioning in granitic melt-vapour systems. Trans Inst Mining Met (inpress)
Manning DAC, Hamilton DL, Henderson CMB, Dempsey M (1980) The probable occurrence of interstitial Al in hydrous, F-bearing and F-free aluminosilicate melts. Contrib Mineral Petrol 75:257–262
Nekrasov IY, Epel'baum MB, Sobolev VP (1980) Partition of tin between melt and chloride fluid in the granite-SnO-SnO2-fluid system. Dokl Akad Nauk SSSR (Earth Sci Sect) 252:165–168
Neumann GM (1973) Thermodynamics of heterogeneous gas equilibria, 5. Gas phase composition and chemical transport reactions in the tungsten-halogen-oxygen system. Z Metallk 64:26–32
Norman JH, Staley HG (1965) Thermodynamics of the dimerisation and trimerisation of gaseous tungsten trioxide and molybdenum trioxide. J Chem Phys 43:3804–3806
Oxtoby S, Hamilton DL (1978) The discrete association of water with Na2O and SiO2 in NaAl silicate melts. Contrib Mineral Petrol 66:185–188
Parish RV (1966) The inorganic chemistry of W. Advances in Inorganic Chemistry and Radiochemistry 9:315–354
Prigent J, Caillet P (1963) Sur les chlorotungstates alcalins non stoechiometriques. CR Acad Sci Ser D 256:2184–2185
Rollinson CL (1973) Chromium, molybdenum and tungsten. In: Bailar JC, Emeleus HJ, Nyholm S, Trotman-Dickenson AF (eds) Comprehensive inorganic chemistry, 3:742–769, Pergamon Press, Oxford
Schmitz-Dumont O, Bruns I, Heckmann I (1953) Die Systeme Alkali-fluorid/Wolfram(VI)-oxyd. Z Anorg Allg Chem 271:347–356
Souchay P (1942) Contribution à l'étude des hétéropolyacides tungstiques (1). Discussions sur l'acidité maxima de certains entre eux. Bull Soc Chim 5e ser 9:289–314
Stemprok M (1982) Tin-fluorine relationships in ore-bearing assemblages. In: Evans AM (ed) Metallization associated with acid magmatism, 6:321–337, John Wiley, New York
Stemprok M, Voldan J (1982) Solubility of tungstic oxide in granitic melts. Vesn ustred Ustavu geol CAV 57:329–340
Tuttle OF, Bowen NL (1958) Origin of granite in the light of experimental studies in the system NaAlSi3O8-KAlSi3O8-SiO2-H2O. Mem Geol Soc Am 74
Tytko K-H, Glemser O (1976) Isopolymolybdates and isopolytungstates. Adv Inorg Chem Radiochem 19:239–315
Watson EB, Capobianco CJ (1981) Phosphorus and the rare earth elements in felsic magmas: an assessment of the role of apatite. Geochim Cosmochim Acta 45:2349–2358
Wesolowski D, Drummond SE, Mesmer RE, Ohmoto H (in prep.) Hydrolysis equilibria of tungsten (VI) in aqueous sodium chloride solutions to 300° C. Submitted to Progr Inorg Chem
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Manning, D.A.C., Henderson, P. The behaviour of tungsten in granitic melt-vapour systems. Contr. Mineral. and Petrol. 86, 286–293 (1984). https://doi.org/10.1007/BF00373674
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00373674