Summary
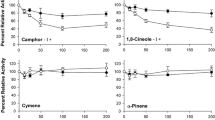
Phenolic glycosides, commonly occurring allelochemicals in the plant family Salicaceae, are differentially toxic to subspecies of the eastern tiger swallowtail and responsible for striking differences in the abilities of Papilio glaucus canadensis and P.g. glaucus to utilize the Salicaceae as food plants. This research was designed to test the hypothesis that particularly high esterase activity confers resistance to phenolic glycosides in P.g. canadensis. I conducted larval survival trials in which the phenolic glycosides salicortin and tremulacin were administered with and without inhibitors of the major detoxication enzymes. Results for P.g. canadensis showed that when esterases were inhibited, toxicity of the phenolic glycosides increased greatly. None of the inhibitors significantly increased toxicity of the compounds to P.g. glaucus. I also conducted in vitro assays of the major detoxication enzymes (polysubstrate monooxygenases, esterases, and glutathione transferases) in larval midguts. Soluble esterase activity was 3-fold higher in P.g. canadensis than in P.g. glaucus. Moreover, esterase activity was inducible by prior consumption of phenolic glycosides in P.g. canadensis but not in P.g. glaucus. Glutathione transferases may also be involved in the terminal metabolism of phenolic glycosides. This is the first verified case of detoxication of an allelochemical by esterase enzymes in herbivores. The biochemical adaptation has played an important role in the evolution of food plant preferences in P. glaucus subspecies.
Similar content being viewed by others
References
Ahmad S (ed) (1983) Herbivorous insects: host-seeking behavior and mechanisms. Academic Press, New York
Barbosa P, Schultz JC (eds) (1987) Insect outbreaks. Academic Press, New York
Brattsten LB (1987) Metabolic insecticide defenses in the boll weevil compared to those in a resistance-prone species. Pest Biochem Physiol 27:1–12
Brattsten LB, Price SL, Gunderson CA (1980) Microsomal oxidases in midgut and fatbody tissues of a boradly herbivorous insect larva, Spodoptera eridania Cramer (Noctuidae). Comp Biochem Physiol 66C:231–237
Brower LP (1958) Larval foodplant specificity in butterflies of the Papilio glaucus group. Lepid News 12:103–114
Bull DL, Ivie GW, Beier RC, Pryor NW (1986) In vitro metabolism of a linear furanocoumarin (8-methoxypsoralen, xanthotoxin) by mixed-function oxidases of larvae of black swallowtail butterfly and fall armyworm. J Chem Ecol 12:885–892
Denno RF, McClure MS (eds) (1983) Variable plants and herbivores in natural and managed systems. Academic Press, New York
Feeny P, Rosenberry L, Carter M (1983) Chemical aspects of oviposition behavior in butterflies. In: Ahmad S (ed) Herbivorous insects: host-seeking behavior and mechanisms. Academic Press, New York, pp 27–76
Gould F (1984) Mixed function oxidases and herbivore polyphagy: the devil's advocate position. Ecol Entomol 9:29–34
Hagen RH (1986) The evolution of host-plant use by the tiger swallowtail butterfly, Papilio glaucus. Dissertation. Cornell University, Ithaca, New York
Hancock DL (1983) Classification of the Papilionidae (Lepidoptera): a phylogenetic approach. Smithersia 2:1–48
Hansen LG, Hodgson E (1971) Biochemical characteristics of insect microsomes. N- and O-demethylation. Biochem Pharmacol 20:1569–1578
Hedin PA, Parrott WL, Jenkins JN, Mulrooney JE, Menn JJ (1988) Elucidating mechanisms of tobacco budworm resistance to allelochemicals by dietary tests with insecticide synergists. Pest Biochem Physiol 32:55–61
Jao LT, Casida JE (1974) Insect pyrethroid-hydrolyzing esterases. Pest Biochem Physiol 4:465–472
Lindroth RL (1989) Host plant alteration of detoxication activity in Papilio glaucus glaucus. ent Exp Appl 50:29–35
Lindroth RL (1988) Hydrolysis of phenolic glycosides by midgut β-glucosidases in Papilio glaucus subspecies. Insect Biochem 18:789–792
Lindroth RL, Peterson SS (1988) Effects of plant phenols on performance of southern armyworm larvae. Oecologia 75:185–189
Lindroth RL, Scriber JM, Hsia MTS (1988) Chemical ecology of the tiger swallowtail: mediation of host use by phenolic glycosides. Ecology 69:814–822
Marty MA, Krieger RI (1984) Metabolism of uscharidin, a milkweed cardenolide, by tissue homogenates of monarch butterfly larvae Danaus plexippus L. J Chem Ecol 10:945–956
Mattes BR, Clausen TP, Reichardt PB (1987) Volatile constituents of balsam poplar: the phenol glycoside connection. Phytochemistry 26:1361–1366
Mouchès C, Pasteur N, Bergé JB, Hyrien O, Raymond M, de Saint Vincent BR, de Silvestri M, Georghiou GP (1986) Amplification of an esterase gene is responsible for insecticide resistance in a California Culex mosquito. Science 233:778–780
Neal RA (1980) Metabolism of toxic substances. In: Doull J, Klaassen CD, Amdur MO (eds) Casarett and Doull's toxicology: the basic science of poisons. Macmillan, New York, pp 56–69
Nitao JK (1989) Biochemical adaptations in a specialist herbivore for feeding on furanocoumarin-containing plants. Ecology 70:629–635
Raffa KF, Priester TM (1985) Synergists as research tools and control agents in agriculture. J Agric Entomol 2:27–45
Rosenthal GA, Janzen DH (eds) (1979) Herbivores. Their interaction with secondary plant metabolites. Academic Press, New York
Schacterle GR, Pollack RL (1973) A simplified method for quantitative assay of small amounts of protein in biologic material. Anal Biochem 51:654–655
Scriber JM (1988) Tale of the tiger: biogeography, binomial classification, and breakfast choices in the Papilio glaucus complex of butterflies. In: Spencer KC (ed) Chemical mediation of coevolution. Academic Press, New York, pp 241–301
Terriere LC (1984) Induction of detoxication enzymes in insects. Annu Rev Entomol 29:71–88
Welling W, DeVries JW (1985) Synergism of organophosphorus insecticides by diethyl maleate and related compounds in house flies. Pest Biochem Physiol 23:358–369
Wilkinson L (1987) SYSTAT: the system for statistics. SYSTAT Inc, Evanston Illinois
Yu SJ (1986) Consequences of induction of foreign compound-metabolizing enzymes in insects. In: Brattsten LB, Ahmad S (eds) Molecular aspects of insect-plant associations. Plenum Press, New York, pp 153–174
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindroth, R.L. Biochemical detoxication: mechanism of differential tiger swallowtail tolerance to phenolic glycosides. Oecologia 81, 219–224 (1989). https://doi.org/10.1007/BF00379809
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00379809