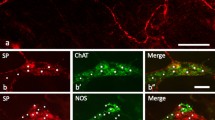
Summary
The present work was undertaken to determine by immunocytochemical methods which of the putative enteric neurotransmitters are contained in axons supplying the guinea-pig taenia coli and what proportion of axons is accounted for by the presence of these substances. Numerous fibres displayed immunoreactivity for dynorphin (DYN), enkephalin (ENK), γ-aminobutyric acid (GABA), nitric oxide synthase (NOS), substance P (SP) and vasoactive intestinal peptide (VIP), but, in contrast to other gut regions, fibres showing immunoreactivity for gastrin-releasing peptide, galanin and neuropeptide Y were rare in the taenia. Fibres reactive for calbindin, calcitonin gene-related peptide, cholecystokinin, 5-hydroxytryptamine and somatostatin were also rare. Tyrosine hydroxylase-like immunoreactivity (TH-LI) was present in numerous fibres that disappeared after extrinsic denervation, a procedure that did not detectably affect any of the other major groups of fibres. Simultaneous staining of extrinsically denervated preparations revealed that SP-LI and VIP-LI were located in separate fibres, and ultrastructural studies showed these to be 58% and 33% of intrinsic fibres supplying the muscle. Immunoreactivity for the general marker, neuron-specific enolase, was located in 95–98% of axons. ENK-LI and DYN-LI were in the same axons, and similar proportions of the fibres with either SP-LI or VIP-LI, about 85%, contained immunoreactivity for ENK and DYN. All VIP-LI fibres, but no SP-LI fibres, were reactive for NOS. The results imply that the taenia of the guinea-pig caecum is innervated by two major groups of enteric neurons: (i) excitatory neurons that contain ACh, SP, other tachykinins, and, in most cases, DYN-LI and ENK-LI; and (ii) inhibitory neurons that contain NOS-LI, VIP-LI, in most cases, the two opioids and, quite probably, ATP as a transmitter. GABA-LI is contained in a smaller population of intrinsic axons. Even though the taenia represents one of the simplest tissues for examining transmission from enteric neurons to intestinal muscle, it shares some of the complexity of other regions, in that four major axon types supply the muscle and both the enteric excitatory and enteric inhibitory neurons contain multiple transmitters.
Similar content being viewed by others
References
Angel F, Go VLW, Schmalz PF, Szurszewski JH (1983) Vasoactive intestinal polypeptide: a putative transmitter in the canine gastric muscularis mucosae. J Physiol 341:641–654
Bartho L, Holzer P (1985) Search for a physiological role of substance P in gastrointestinal motility. Neuroscience 16:1–32
Bennett MR (1966) Transmission from intramural excitatory nerves to the smooth muscle cells of the guinea-pig taenia coli. J Physiol 185:132–147
Bennett MR, Burnstock G, Holman ME (1966) Transmission from intramural inhibitory nerves to the smooth muscle cells of the guinea-pig taenia coli. J Physiol 182:541–558
Bennett MR, Rogers DC (1967) A study of the innervation of the taenia coli. J Cell Biol 33:573–596
Bredt DS, Hwang PM, Snyder SH (1990) Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347:768–770
Bulbring E (1955) Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol 128:200–221
Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG (1990) Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345:346–347
Burnstock G (1979) Past and current evidence for the purinergic nerve hypothesis. In: Baer HP, Drummond GI (ed) Physiological and regulatory functions of adenosine and adenine nucleotides. Raven Press, New York, pp 3–32
Burnstock G (1981) Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol 313:1–35
Burnstock G, Campbell G, Bennett M, Holman ME (1964) Innervation of the guinea-pig taenia coli: are there intrinsic inhibitory nerves which are distinct from sympathetic nerves. Int J Neuropharmacol 3:163–166
Burnstock G, Campbell G, Rand MJ (1966) The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol 182:504–526
Cocks T, Burnstock G (1979) Effects of neuronal polypeptides on intestinal smooth muscle: A comparison with non-adrenergic, non-cholinergic nerve stimulation and ATP. Eur J Pharmacol 54:251–259
Costa M, Furness JB (1983) The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guineapig small intestine. Neuroscience 8:665–676
Costa M, Furness JB, Llewellyn-Smith IJ, Davies B, Oliver J (1980) An immunohistochemical study of the projections of somatostatin-containing neurons in the guinea-pig intestine. Neuroscience 5:841–852
Costa M, Furness JB, Cuello AC, Verhofstad AAJ, Steinbusch HWM, Elde RP (1982) Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their visualization and reactions to drug treatment. Neuroscience 71:351–363
Costa M, Furness JB, Yanaihara N, Yanaihara C, Moody TW (1984) Distribution and projections of neurons with immunore-activity for both gastrin-releasing peptide and bombesin in the guinea-pig small intestine. Cell Tissue Res 235:285–293
Cuello AC, Galfre G, Milstein C (1979) Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 76:3532–3536
Daniel EE, Furness JB, Costa M, Belbeck L (1987) The projections of chemically identified nerve fibres in canine ileum. Cell Tissue Res 247:377–384
Ekblad E, Winther C, Ekman R, Håkanson R, Sundler F (1987) Projections of peptide-containing neurons in rat small intestine. Neuroscience 20:169–188
Ekblad E, Håkanson R, Sundler F (1991) Microanatomy and chemical coding of peptide containing neurons in the digestive tract. In: Daniel EE (ed) Neuropeptide function in the gastrointestinal tract. CRC Press, Boston, pp 131–179
Fahrenkrug J (1979) Vasoactive intestinal polypeptide: measurement, distribution and putative transmitter function. Digestion 19:149–169
Furness JB, Costa M (1979) Projections of intestinal neurons showing immunoreactivity for vasoactive intestinal polypeptide are consistent with these neurons being the enteric inhibitory neurons. Neurosci Lett 15:199–204
Furness JB, Costa M (1987) The Enteric Nervous System, Churchill Livingstone, Edinburgh London Melbourne
Furness JB, Costa M, Walsh JH (1981) Evidence for and significance of the projection of VIP neurons from the myenteric plexus to the taenia coli in the guinea-pig. Gastroenterology 80:1557–1561
Furness JB, Costa M, Emson PC, Håkanson R, Moghimzadeh E, Sundler F, Taylor IL, Chance RE (1983a) Distribution, pathways and reactions to drug treatment of nerves with neuropeptide Y- and pancreatic polypeptide-like immunoreactivity in the guinea-pig digestive tract. Cell tissue Res 234:71–92
Furness JB, Costa M, Miller RJ (1983b) Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience 8:653–664
Furness JB, Costa M, Rokaeus A, McDonald TJ, Brooks B (1987) Galanin-immunoreactive neurons in the guinea-pig small intestine: their projections and relationships to other enteric neurons. Cell Tissue Res 250:607–615
Furness JB, Keast JR, Pompolo S, Bornstein JC, Costa M, Emson PC, Lawson DEM (1988a) Immunohistochemical evidence for the presence of calcium binding protein in enteric neurons. Cell Tissue Res 252:79–87
Furness JB, Llewellyn-Smith IJ, Bornstein JC, Costa M (1988b) Chemical neuroanatomy and the analysis of neuronal circuitry in the enteric nervous system. In: Björklund A, Hökfelt T, Owman C (eds) Handbook of Chemical Neuroanatomy, vol. 6, The peripheral nervous system. Elsevier, Amsterdam, pp 161–218
Furness JB, Trussell DC, Pompolo S, Bornstein JC, Maley BE, Storm-Mathisen J (1989) Shapes and projections of neurons with immunoreactivity for gamma-aminobutyric acid in the guinea-pig small intestine. Cell Tissue Res 256:293–301
Furness JB, Lloyd KCK, Sternini C, Walsh JH (1990) Projections of substance P, vasoactive intestinal peptide and tyrosine hydroxylase immunoreactive nerve fibres in the canine intestine, with special reference to the innervation of the circular muscle. Arch Histol Cytol 53:129–140
Furness JB, Bornstein JC, Murphy R, Pompolo S (1992) Roles of peptides in transmission in the enteric nervous system. Trends Neurosci 15:66–71
Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S (1985) Co-localization of calcitonin gene related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea-pigs. Neurosci Lett 57:125–130
Grider JR, Makhlouf GM (1986) Colonic peristaltic reflex: identification of vasoactive intestinal peptide as a mediator of descending relaxation. Am J Physiol 251:40–45
Grider JR, Cable MB, Bitar KN, Said SI, Makhlouf GM (1985) Vasoactive intestinal polypeptide. Relaxant neurotransmitter in taenia coli of the guinea pig. Gastroenterology 89:36–42
Hoyle CHV, Kamm MA, Burnstock G, Lennard-Jones JE (1990a) Enkephalins modulate inhibitory neuromuscular transmission in circular muscle of human colon via delta-opioid receptors. J Physiol 431:465–478
Hoyle CHV, Knight GE, Burnstock G (1990b) Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol 99:617–621
Jessen KR, Saffrey MJ, Van Noorden S, Bloom SR, Polak JM, Burnstock G (1980) Immunohistochemical studies of the enteric nervous system in tissue culture and in situ: Localization of vasoactive intestinal polypeptide (VIP), substance P and enkephalin immunoreactive nerves in the guinea-pig gut. Neuroscience 5:1717–1735
Jessen KR, Hills JM, Dennison ME, Mirsky R (1983) γ-Aminobutyrate as an autonomic neurotransmitter: Release and uptake of [3H]γ-aminobutyrate in guinea pig large intestine and cultured enteric neurons using physiological methods and electron microscopic autoradiography. Neuroscience 10:1427–1442
Jessen KR, Mirsky R, Hills JM (1987) GABA as an autonomic neurotransmitter: studies on intrinsic GABAergic neurons in the myenteric plexus of the gut. Trends in Neurosci 10:255–262
Johnson SM, Williams JT, Costa M, Furness JB (1987) Naloxone-induced depolarization and synaptic activation of myenteric neurones in morphine dependent guinea-pig ileum. Neuroscience 21:595–602
Knudsen MA, Tottrup A (1991) The inhibitory innervation of guinea-pig taenia coli. J Gastrointest Motility 3:186
Leander S, Håkanson R, Sundler F (1981a) Nerves containing substance P, vasoactive intestinal polypeptide, enkephalin or somatostatin in the guinea-pig taenia coli. Cell Tissue Res 215:21–39
Leander S, Håkanson R, Rosell S, Folkers K, Sundler F, Tomqvist K (1981b) A specific substance P antagonist blocks smooth muscle contractions induced by non-cholinergic, non-adrenergic nerve stimulation. Nature 294:467–469
Li CG, Rand MJ (1989) Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol 16:933–938
Li CG, Rand MJ (1990) Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic non-cholinergic inhibitory transmission to the rat gastric fundus. Eur J Pharmacol 191:303–309
Llewellyn-Smith IJ, Costa M, Furness JB (1985) Light and electron microscope immunocytochemistry of the same nerves from whole mount preparations. J Histochem Cytochem 33:856–867
Llewellyn-Smith IJ, Furness JB, Gibbins IL, Costa M (1988) Quantitative ultrastructural analysis of enkephalin-, substance P-, and VIP-immunoreactive nerve fibers in the circular muscle of the guinea pig small intestine. J Comp Neurol 139:148
Macarrone C, Jarrott B (1985) Differences in regional brain concentrations of neuropeptide Y in spontaneously hypertensive (SH) and Wistar-Kyoto (WKY) rats. Brain Res 345:165–169
MacKenzie I, Burnstock G (1980) Evidence against vasoactive intestinal polypeptide being the non-adrenergic, non-cholinergic inhibitory transmitter released from nerves supplying the smooth muscle of the guinea-pig taenia coli. Eur J Pharmacol 67:255–264
Makhlouf GM, Grider JR, Schubert ML (1989) Identification of physiological function of gut peptides. In: Makhlouf GM (ed) Handbook of Physiology, Sect. 6: The Gastrointestinal System, vol. II. American Physiological Society Washington DC, pp 123–131
Maley B, Newton B (1985) Immunohistochemistry of γ-aminobutyric acid in the cat nucleus tractus solitarius. Brain Res 330:364–368
Messenger JP, Furness JP (1990) Projections of chemically-specified neurons in the guinea-pig colon. Arch Histol Cytol 53:467–495
Morris JL, Gibbins IL, Furness JB, Costa M, Murphy R (1985) Co-localization of NPY, VIP and dynorphin in non-adrenergic axons of the guinea-pig uterine artery. Neurosci Lett 62:31–37
Morris JL, Gibbins IL, Campbell G, Murphy R, Furness JB, Costa M (1986) Innervation of the large arteries and heart of the toad (Bufo marinus) by adrenergic and peptide-containing neurons. Cell Tissue Res 243:171–184
Niel JP, Bywater RAR, Taylor GS (1983) Effect of substance P of non-cholinergic fast and slow post-stimulus depolarisation in the guinea-pig ileum. J Auton Nerv Syst 9:573–584
Pompolo S, Furness JB (1988) Ultrastructure and synaptic relationships of calbindin-reactive type II neurons, in myenteric ganglia of guinea-pig small intestine. J Neurocytol 17:771–782
Pompolo S, Furness JB (1990) Ultrastructure and synaptology of neurons immunoreactive for gamma-aminobutyric acid in the myenteric plexus of the guinea pig small intestine. J Neurocytol 19:539–549
Rchfeld JF, Larsson L-I (1979) The predominating molecular form of gastrin and cholecystokinin in the gut is a small peptide corresponding to their COOH-terminal tetrapeptide amide. Acta Physiol Scand 105:117–119
Ronai AZ, Kardos J, Simonyi M (1987) Potent inhibitory GABAB receptors in stimulated guinea pig taenia coli. Neuropharmacology 26:1623–1627
Steele PA, Brookes SJH, Costa M (1991) Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience 45:227–239
Shuttleworth CWR, Murphy R, Furness JB, Pompolo S (1991) Comparison of the presence and actions of substance P and neurokinin A in guinea-pig taenia coli. Neuropeptides 19:23–34
Taylor GS, Bywater RAR (1986) Antagonism of non-cholinergic excitatory junction potentials in the guinea-pig ileum by a substance P analogue antagonist. Neurosci Lett 63:23–26
Wattchow DA, Furness JB, Costa M (1988) Distribution and coexistence of peptides in nerve fibers of the external muscle of the gastrointestinal tract. Gastroenterology 95:32–41
Yamauchi A (1964) Electron microscope studies of the autonomic neuromuscular junction in the taenia coli of guinea-pig. Acta Anat Nippon 39:22–30
Young HM, Furness JB, Shuttleworth CWR, Bredt DS, Snyder SH (1992) Co-localization of nitric oxide synthase immunoreactivity and NADPH diaphorase staining in neurons of the guinea-pig intestine. Histochemistry 97:375–378
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Furness, J.B., Pompolo, S., Shuttleworth, C.W.R. et al. Light- and electron-microscopic immunochemical analysis of nerve fibre types innervating the taenia of the guinea-pig caecum. Cell Tissue Res. 270, 125–137 (1992). https://doi.org/10.1007/BF00381887
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00381887