Abstract
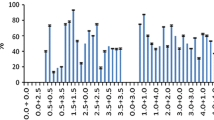
Using callus derived from immature embryos, regeneration of viable plants was obtained in soybean (Glycine max (L.) Merr.). Depending on the composition of the medium, regeneration occurred via embryogenesis or via organogenesis. Embryogenesis resulted when embryos were plated on Murashige and Skoog (MS) medium containing 43 μM α-naphthaleneacetic acid. In work with the cultivar Williams 82, the addition of 5.0 μM thiamine HCl increased embryogenesis from 33% to 58% of the embryos plated. Addition of 30 μM nicotinic acid to the MS medium enhanced embryogenesis further to 76%. Organogenesis was obtained when medium containing 13.3 μM 6-benzylaminopurine, 0.2 μM and α-naphthaleneacetic acid and four times the normal concentration of MS minor salts was used. Histological studies of these cultures confirmed the organogenic and embryogenic nature of the cultures, by demonstrating the formation of shoot buds and somatic embryos, respectively. Similar responses were obtained in all 54 genotypes tested in this manner. The cultures retained the ability to regenerate complete plants for at least 12 months and 12–15 subcultures. Seeds have been obtained from several regenerated plants and when grown in the field these produced normal-appearing fertile plants.
Similar content being viewed by others
Abbreviations
- BAP:
-
6-benzylaminopurine
- 2,4-D:
-
2,4-dichlorophenoxyacetio acid
- GA3 :
-
gibberellic acid
- IAA:
-
indole-3-acetic acid
- IBA:
-
indole-3-butyric acid
- MS:
-
Murashige and Shoog (1962) medium
- NAA:
-
α-naphthaleneacetic acid
- picloram:
-
4-amino-3,5,6-trichloropicolinic acid
References
Armstrong, C.L., Green, C.E. (1985) Establishment and maintenance of friable, embryogenic maize callus and the involvement of l-proline. Planta 164, 207–214
Barwale, U.B., Meyer, M.M., Widholm, J.M. (1986) Theor. Appl. Genet. (in press)
Beversdorf, W.D., Bingham, E.T. (1977) Degrees of differentiation obtained in tissue cultures of Glycine species. Corp Sci. 17, 307–311
Cheng, T.Y., Saka, T., Voqui-Dinh, T.H. (1980) Plant regeneration from soybean cotyledonary node segments in culture. Plant Sci. Lett. 19, 91–99
Christianson, M.L., Warnick, D.A., Carlson, P.S. (1983) A morphogenetically competent soybean suspension culture. Science 222, 632–634
Dos Santos, A.V.P., Cutter, E.G., Davey, M.R. (1983) Origin and development of somatic embryos in Medicago sativa L. (alfalfa). Protoplasma 117, 107–115
Gamborg, O.L., Miller, R.A., Ojima, K. (1968) Exp. Cell Res. 50, 148–151
Gamborg, O.L., Davis, B.P., Stahlhut, R.W. (1983) Somatic embryogenesis in cell cultures of Glycine species. Plant Cell Rep. 2, 209–212
Grant, J. (1984) Plant regeneration from cotyledonary tissue of Glycine canescens, a perennial wild relative of soybean. Plant Cell Tissue Organ Cult 3, 169–173
Hoagland, D.R., Arnon, D.I. (1950) The water-culture method for growing plants without soil. California Agric. Exp. Sta. Bull. No. 347
Johansen, D.A. (1940) Plant microtechnique. McGraw-Hill Publ., New York London
Kameya, T., Widholm, J.M. (1981) Plant regeneration from hypocotyl sections of Glycine species. Plant Sci. Lett. 21, 289–294
Kimball, S.L., Bingham, E.T. (1973) Adventitious bud development of soybean hypocotyl sections in culture. Crop Sci. 13, 758–760
Lippmann, B., Lippmann, G. (1984) Induction of somatic embryos in cotyledonary tissue of soybean Glycine max L. Merr. Plant Cell Rep. 3, 215–218
Murashige, T., Skoog, F. (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant 15, 473–497
Newell, C.A., Luu, H.T. (1985) Protoplast culture and plant regeneration in Glycine canescens F. J. Herm. Plant Cell Tissue Organ Cult. 4, 145–149
Oswald, T.H., Smith, R.A., Phillips, D.V. (1977) Physiol. Plant. 39, 129–134
Phillips, G.C., Collins, G.B. (1981) Induction and development of somatic embryos from cell suspension cultures of soybean. Plant Cell Tissue Organ Cult 1, 123–129
Saka H.T., Voqui-Dinh, T.H., Cheng, T.Y. (1980) Stimulation of multiple shoot formation on soybean stem nodes in cultures. Plant Sci. Lett. 19, 193–201
Widholm, J.M., Rick, S. (1983) Shoot regeneration from Glycine canescens tissue cultures. Plant Cell Rep. 2, 19–20
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barwale, U.B., Kerns, H.R. & Widholm, J.M. Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta 167, 473–481 (1986). https://doi.org/10.1007/BF00391223
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391223